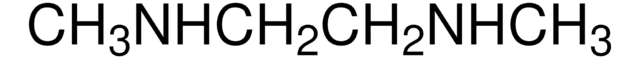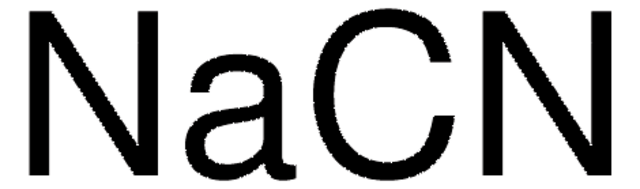270032
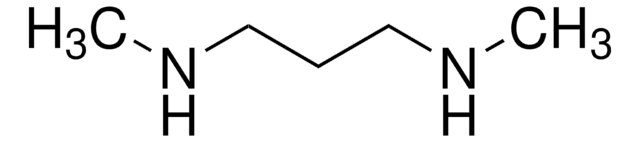
N,N′-Dimethylethylenediamine
85%
Synonym(s):
1,2-Bis(methylamino)ethane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3NHCH2CH2NHCH3
CAS Number:
Molecular Weight:
88.15
Beilstein:
878142
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
85%
form
liquid
refractive index
n20/D 1.431 (lit.)
bp
119 °C (lit.)
density
0.819 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
CNCCNC
InChI
1S/C4H12N2/c1-5-3-4-6-2/h5-6H,3-4H2,1-2H3
InChI key
KVKFRMCSXWQSNT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N′-Dimethylethylenediamine was used to enhance CO2 adsorption. It was incorporated into H3[(Cu4Cl)3(BTTri)8 (CuBTTri; H3BTTri = 1,3,5-tri(1H-1,2,3-triazol-4-yl)benzene), a water-stable, triazolate-bridged framework, to form a metal–organic framework for CO2 separation.
Other Notes
remainder N-Methylethylenediamine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
78.8 °F - closed cup
Flash Point(C)
26 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enhanced carbon dioxide capture upon incorporation of N, N?-dimethylethylenediamine in the metal?organic framework CuBTTri.
McDonald TM, et al.
Chemical Science, 2(10), 2022-2028 (2011)
J T Hwang et al.
Nucleic acids research, 27(19), 3805-3810 (1999-09-11)
The formal C1'-oxidation product, 2-deoxyribonolactone, is formed as a result of DNA damage induced via a variety of agents, including gamma-radiolysis and the enediyne antitumor antibiotics. This alkaline labile lesion may also be an intermediate during DNA damage induced by
Deprotection of N(in)-formyl group on tryptophan by application of new reagent, 1,2-dimethylethylendiamine in aqueous solution.
Takenao Odagami et al.
Advances in experimental medicine and biology, 611, 159-160 (2009-04-30)
Kim A Lennox et al.
Oligonucleotides, 16(1), 26-42 (2006-04-06)
A wide variety of modified oligonucleotides have been tested as antisense agents. Each chemical modification produces a distinct profile of potency, toxicity, and specificity. Novel cationic phosphoramidate-modified antisense oligonucleotides have been developed recently that have unique and interesting properties. We
R Biyik et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 68(2), 394-398 (2007-02-13)
Cu(2+) doped single crystals of [Zn(sac)2(dmen)] (sac: saccharinate, dmen: N,N'-dimethylethylendiamine) and [Zn(sac)2(paen)], (paen: N,N'-bis(3-propylamine)ethylendiamine) complexes have been investigated by electron paramagnetic resonance (EPR) technique. Detailed investigations of the EPR spectra indicate that Cu(2+) ion substitute with Zn(2+) ion and forms
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service