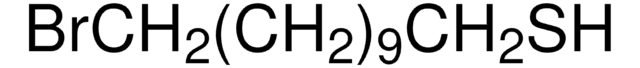241776
Potassium thioacetate
98%
Synonym(s):
Ethanethioic acid potassium salt, Thioacetic acid potassium salt, Thiolacetic acid potassium salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
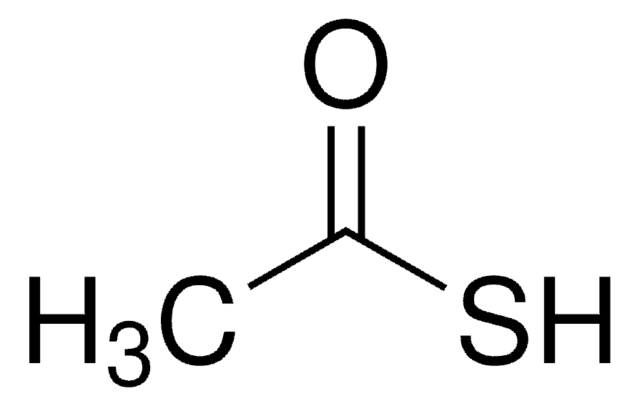
CH3COSK
CAS Number:
Molecular Weight:
114.21
Beilstein:
4428862
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Assay:
98%
form:
solid
Recommended Products
Quality Level
Assay
98%
form
solid
mp
173-176 °C (lit.)
SMILES string
[K+].CC([S-])=O
InChI
1S/C2H4OS.K/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
InChI key
AFNBMGLGYSGFEZ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Potassium thioacetate is a sulfur source in sulfuration reactions and is used as a reagent in nucleophilic substitution and vinylic substitution reactions.
Application
Palladium mediated coupling with aryl halides and triflates leading to S-arylthioacetates and derivatives.
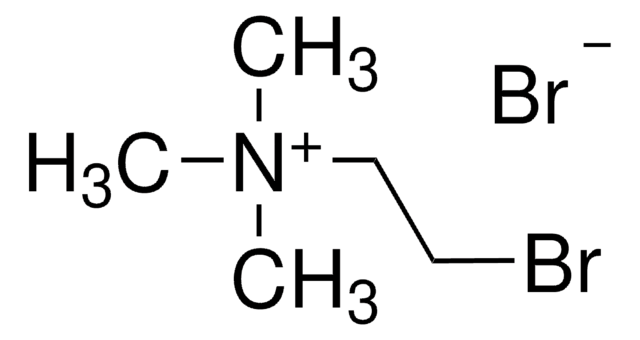
Reagent in the preparation of thiols from halides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Subal Dey et al.
Inorganic chemistry, 57(10), 5939-5947 (2018-05-02)
Reduction of CO2 holds the key to solving two major challenges taunting the society-clean energy and clean environment. There is an urgent need for the development of efficient non-noble metal-based catalysts that can reduce CO2 selectively and efficiently. Unfortunately, activation
Yang Wang et al.
Polymers, 11(6) (2019-06-13)
Photodynamic therapy (PDT) as a non-aggressive therapy with fewer side effects has unique advantages over traditional treatments. However, PDT still has certain limitations in clinical applications, mainly because most photosensitizers utilized in PDT are hydrophobic compounds, which will self-aggregate in
Tetrahedron Letters, 48, 3033-3033 (2007)
Christina Wedemeyer-Exl et al.
Organic & biomolecular chemistry, 5(13), 2119-2128 (2007-06-22)
The thiol-dependent methylation of heptamethyl cob(II)yrinate 8r with methyl iodide and methyl tosylate was explored under a variety of conditions. The interaction of the heptamethyl cob(II)yrinate with a variety of thiols was monitored prior to the addition of the methylating
Ning Shangguan et al.
Journal of the American Chemical Society, 125(26), 7754-7755 (2003-06-26)
A new amide synthesis strategy based on a fundamental mechanistic revision of the reaction of thio acids and organic azides is presented. The data demonstrate that amines are not formed as intermediates in this reaction. Alternative mechanisms proceeding through a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service