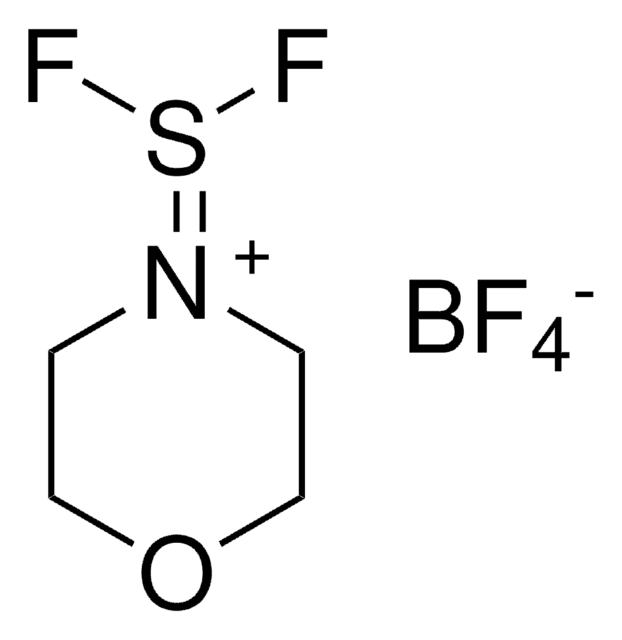
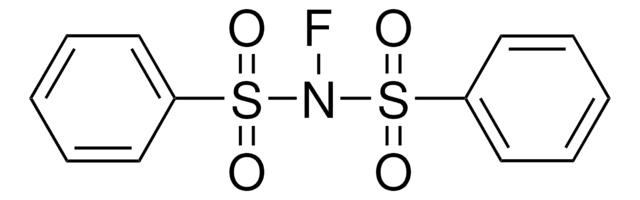
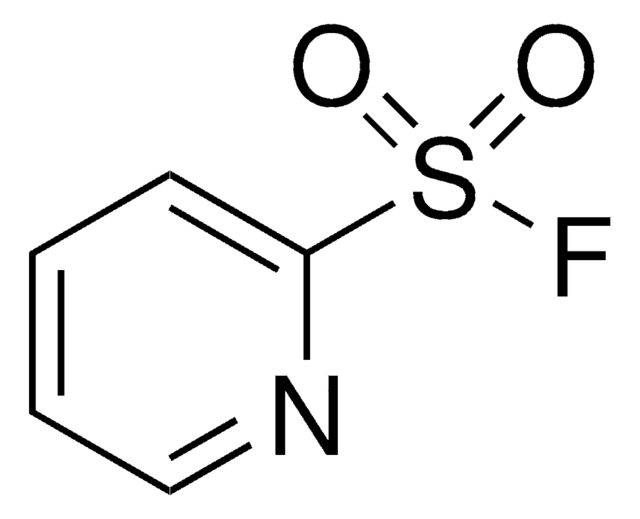
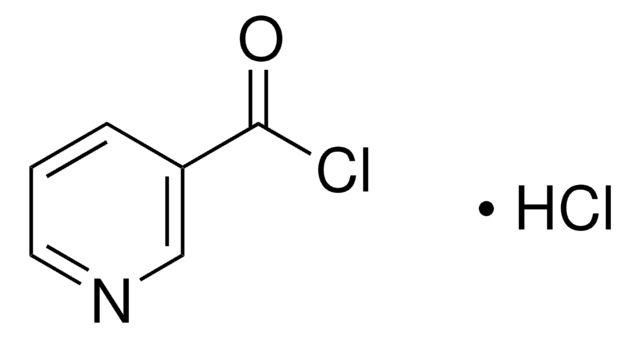
235253
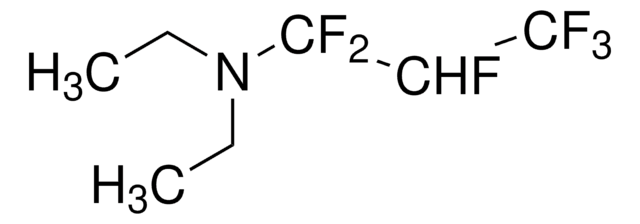
(Diethylamino)sulfur trifluoride
95%
Synonym(s):
DAST, Diethylaminosulfur trifluoride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)2NSF3
CAS Number:
Molecular Weight:
161.19
Beilstein:
1849066
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
bp
30-32 °C/3 mmHg (lit.)
density
1.22 g/mL at 25 °C (lit.)
functional group
amine
storage temp.
2-8°C
SMILES string
CCN(CC)S(F)(F)F
InChI
1S/C4H10F3NS/c1-3-8(4-2)9(5,6)7/h3-4H2,1-2H3
InChI key
CSJLBAMHHLJAAS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Diethylamino sulfur trifluoride (DAST) is a fluorinating reagent used in the synthesis of fluorinated compounds and ring-opening reactions.
Application
- Fluorinating agent: reaction with alcohols and carbonyl compounds, Review
- Review on nucleophilic fluorination.
- Catalyst for Friedel-Crafts allylation using tertiary cyclopropyl silyl ethers and the rearrangement of homoallylic alcohols to unsaturated aldehydes.
- Early introduction of a fluoromethyl group stabilizes the epoxide during further manipulations in the synthesis of 26-fluoro-epothilone.
- Fluorinating agent for a variety of compounds, including thioethers, alkenols, and cyanohydrins.
- Reagent for gem difluorination of ketopipecolinic acids.
replaced by
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Self-react. D - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
73.4 °F
Flash Point(C)
23 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A J Phillips et al.
Organic letters, 2(8), 1165-1168 (2000-05-11)
[formula: see text] A mild and highly efficient cyclization of beta-hydroxy amides to oxazolines is described using DAST and Deoxo-Fluor reagents. A one-pot protocol for the synthesis of oxazoles from beta-hydroxy amides is also presented.
P G Hultin et al.
Carbohydrate research, 322(1-2), 14-25 (2000-01-12)
We have made thioglycoside donors for the 4,6-dideoxy-L-lyxo-hexopyranosyl ('4-deoxy-L-rhamnosyl') and 4-deoxy-4-fluoro-L-rhamnosyl monosaccharide residues. The preparation of the deoxyfluororhamnose was not straightforward, and revealed some unexpected behavior of the diethylaminosulfur trifluoride (DAST) reagent. The new glycosyl donors were used to synthesize
Po-Chiao Lin et al.
The Journal of organic chemistry, 74(11), 4041-4048 (2009-05-16)
When saccharides bearing a sulfur, selenium, or oxygen substituent at the anomeric center and an unprotected hydroxyl group either at C-4 or C-6 were subjected to fluorination with DAST in dichloromethane, a regioselective migration of the anomeric substituent to the
Aurélien Bigot et al.
Organic letters, 13(2), 192-195 (2010-12-15)
The design of a new potent nonsteroidal ecdysone agonist led to the discovery of a diethylaminosulfur trifluoride (DAST)-mediated cyclization of α,α-disubstituted-α-acylaminoketones. The resulting fluorooxazolines can be ring-opened or selectively substituted by a range of nucleophiles to provide in high yields
Jindřich Karban et al.
Organic & biomolecular chemistry, 10(2), 394-403 (2011-11-16)
A complete series of eight 1,6:2,3- and 1,6:3,4-dianhydro-β-D-hexopyranoses were subjected to fluorination with DAST. The 1,6:3,4-dianhydropyranoses yielded solely products of skeletal rearrangement resulting from migration of the tetrahydropyran oxygen (educts of D-altro and D-talo configuration) or of the 1,6-anhydro bridge
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)