235229
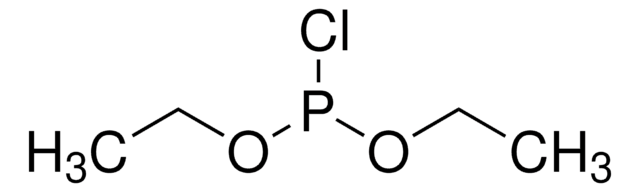
Methyl dichlorophosphite
technical grade
Synonym(s):
Methyl phosphorodichloridite
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OPCl2
CAS Number:
Molecular Weight:
132.91
Beilstein:
1697452
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
liquid
refractive index
n20/D 1.474 (lit.)
bp
93-95 °C (lit.)
mp
−91 °C (lit.)
density
1.376 g/mL at 20 °C (lit.)
SMILES string
COP(Cl)Cl
InChI
1S/CH3Cl2OP/c1-4-5(2)3/h1H3
InChI key
HCSDJECSMANTCX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl dichlorophosphite has been employed in the preparation of:
- phosphonamidate- and phosphonate-linked phosphonopeptides
- 2-(2-iodophenyl)ethyl methyl phosphite derivative of an alcohol
- deoxyoligonucleotides on a polymer support
- cis- and trans-2-oxo-2-propionyl-1,3,2-oxazaphosphorinane
- phosphorodichloridothioates
- oxazaphosphorinanes
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
77.0 °F - closed cup
Flash Point(C)
25 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and enantioselective aldol reaction of a chiral 2-oxo-2-propionyl-1, 3, 2-oxazaphosphorinane.
Gordon NJ and Evans Jr SA.
The Journal of Organic Chemistry, 58(20), 5295-5297 (1993)
Synthesis of deoxyoligonucleotides on a polymer support.
Matteucci MD and Caruthers MH.
Journal of the American Chemical Society, 103(11), 3181-3191 (1981)
Nanyan Fu et al.
Journal of peptide science : an official publication of the European Peptide Society, 12(4), 303-309 (2005-10-26)
A direct method for the preparation of phosphonamidate- and phosphonate-linked phosphonopeptides has been developed. Using this method, both phosphonopeptides were prepared in acceptable yields directly from simple and commercially available chemicals in one-pot reactions of benzyl carbamate, aldehydes, and methyl
Liming Zhang et al.
Journal of the American Chemical Society, 126(41), 13190-13191 (2004-10-14)
A highly efficient, two-step sequence method for the deoxygenation of hydroxyl groups has been developed. The method involves the preparation of the 2-(2-iodophenyl)ethyl methyl phosphite derivative of an alcohol using methyl dichlorophosphite and 2-(2-iodophenyl)ethanol. Treatment of the phosphite intermediate with
Synthetic Communications, 22, 289-289 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service