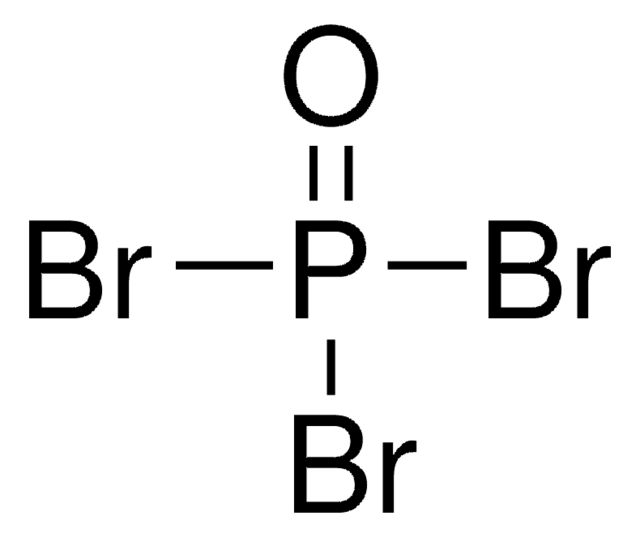226734
Phosphorus pentabromide
95%
Synonym(s):
Pentabromophosphorane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
PBr5
CAS Number:
Molecular Weight:
430.49
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
powder
SMILES string
BrP(Br)(Br)(Br)Br
InChI
1S/Br5P/c1-6(2,3,4)5
InChI key
QRKVRHZNLKTPGF-UHFFFAOYSA-N
Application
Phosphorus pentabromide (PBr5) is a bromination agent generally used to convert alcohols to bromides and in the dibromination of ketones. PBr5 can also be used as the phosphorus source in the preparation of indium phosphide (InP) nanowires.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Identification of potential off-target toxicity liabilities of catechol-O-methyltransferase inhibitors by differential competition capture compound mass spectrometry.
von Kleist L, et al.
Journal of Medicinal Chemistry, 59(10), 4664-4675 (2016)
Synthesis, characterization and photoconductivity of highly crystalline InP nanowires prepared from solid hydrogen phosphide.
Lim T H, et al.
Journal of Materials Chemistry, 19(27), 4852-4856 (2009)
Phosphorus(V) Bromide.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2008)
Highly luminescent hydrogels synthesized by covalent grafting of lanthanide complexes onto PNIPAM via one-pot free radical polymerization.
Li Q F, et al.
Journal of Material Chemistry C, 4(15), 3195-3201 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service