201014
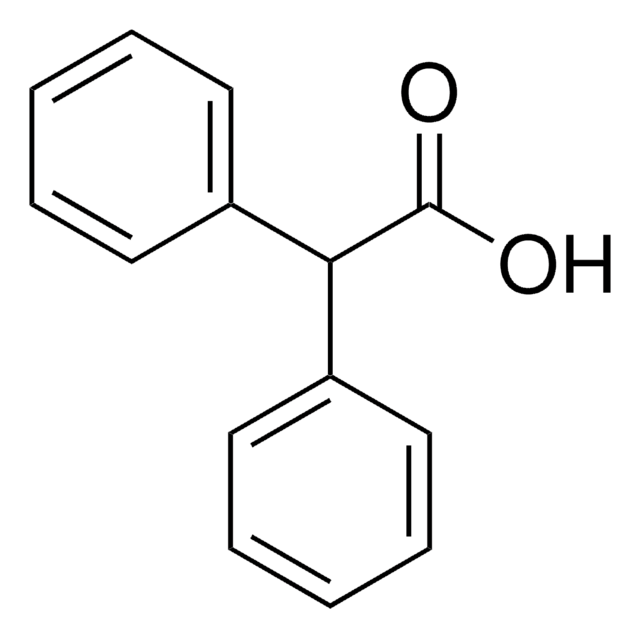
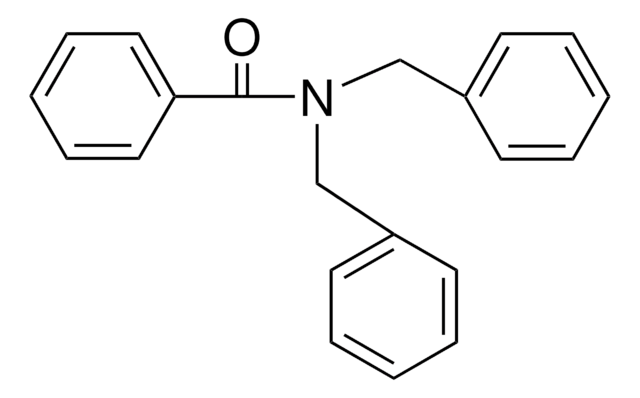
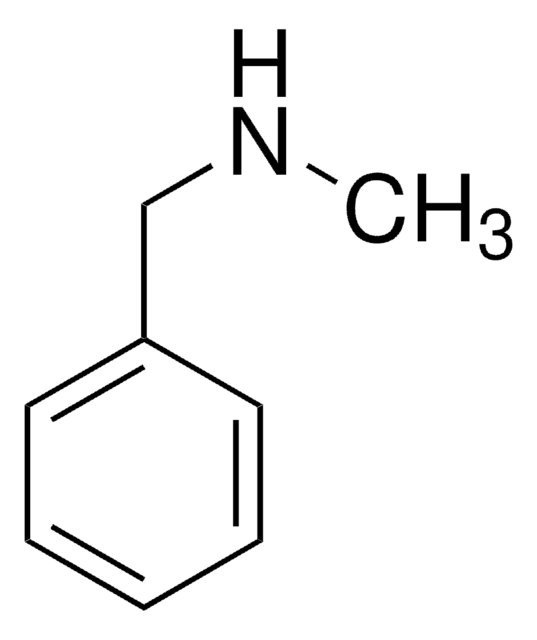
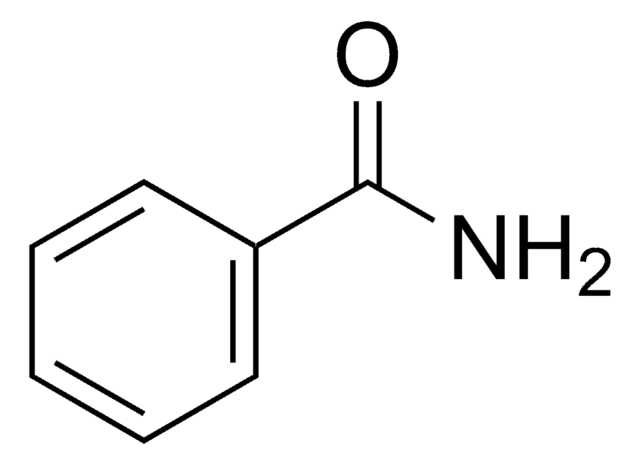
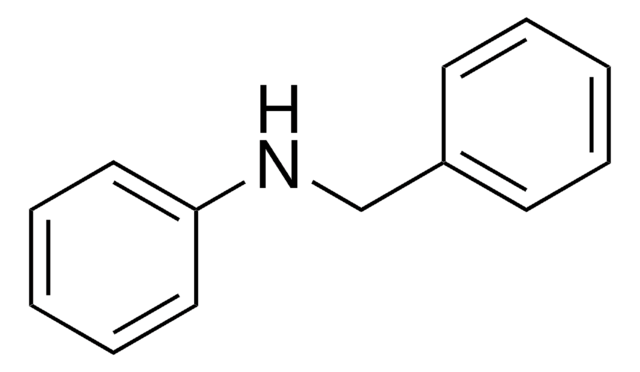
N-Benzylbenzamide
≥98%
Synonym(s):
Benzoic acid benzylamide, N-(Phenylmethyl)benzamide, N-Benzoylbenzylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CONHCH2C6H5
CAS Number:
Molecular Weight:
211.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
mp
104-106 °C (lit.)
solubility
acetone: 25 mg/mL, clear, colorless
functional group
amide
phenyl
SMILES string
O=C(NCc1ccccc1)c2ccccc2
InChI
1S/C14H13NO/c16-14(13-9-5-2-6-10-13)15-11-12-7-3-1-4-8-12/h1-10H,11H2,(H,15,16)
InChI key
LKQUCICFTHBFAL-UHFFFAOYSA-N
General description
N-Benzylbenzamide inhibits the activity of tyrosinase.
Application
A convenient precursor to α-substituted benzylamines and an indicator for the titration of butyllithium and other lithium bases. For references see Aldrichimica Acta .
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aldrichimica Acta, 11, 20-20 (1978)
Yae Eun Chong et al.
Biomedical chromatography : BMC, 33(11), e4653-e4653 (2019-07-20)
Ondansetron, a widely used antiemetic agent, is a P-glycoprotein (P-gp) substrate and therefore expression of P-gp at the blood-brain barrier limits its distribution to the central nervous system (CNS), which was observed to be reversed by coadministration with P-gp inhibitors.
Zsanett Dorkó et al.
Talanta, 162, 167-173 (2016-11-14)
A simple and efficient method is presented for assessing molecularly imprinted polymers (MIP) and other sorbents from the point of view of practical applications. The adsorption isotherms of the compounds, which need to be separated or detected in an application
Sung Jin Cho et al.
Bioorganic & medicinal chemistry letters, 16(10), 2682-2684 (2006-03-04)
A series of potent inhibitors of tyrosinase and their structure-activity relationships are described. N-Benzylbenzamide derivatives (1-21) with hydroxyl(s) were synthesized and tested for their tyrosinase inhibitory activity. With this series, compound 15 provided a potent tyrosinase inhibition: it effectively inhibited
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service