196967
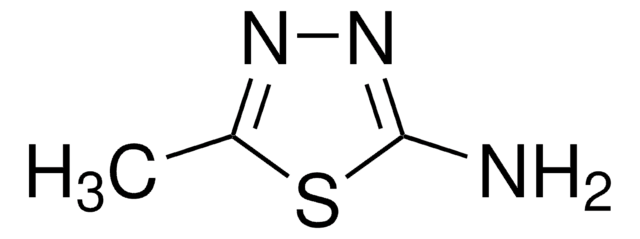
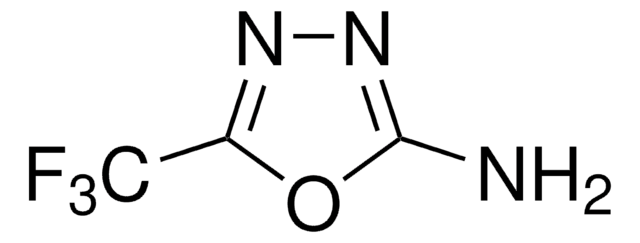
2-Amino-5-trifluoromethyl-1,3,4-thiadiazole
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H2F3N3S
CAS Number:
Molecular Weight:
169.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
225-227 °C (lit.)
solubility
methanol: soluble 0.25 g/10 mL, clear, colorless
SMILES string
Nc1nnc(s1)C(F)(F)F
InChI
1S/C3H2F3N3S/c4-3(5,6)1-8-9-2(7)10-1/h(H2,7,9)
InChI key
LTEUXHSAYOSFGQ-UHFFFAOYSA-N
Application
2-Amino-5-trifluoromethyl-1,3,4-thiadiazole was used in the synthesis of:
- series of 2-sulfonamido/trifluoromethyl-6-(4′-substituted aryl/heteroaryl)imidazo[2,1-b]-1,3,4-thiadiazole derivatives
- 2-trifluoromethyl/sulfonamido-5,6-diarylsubstituted imidazo[2,1-b]-1,3,4-thiadiazole derivatives
- bicyclic bridgehead itrogen heterocycles
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Andanappa K Gadad et al.
Bioorganic & medicinal chemistry, 12(21), 5651-5659 (2004-10-07)
A series of 2-sulfonamido/trifluoromethyl-6-(4'-substituted aryl/heteroaryl)imidazo[2,1-b]-1,3,4-thiadiazole derivatives (II) have been synthesized by reaction of 2-amino-5-sulfonamido/trifluoromethyl-1,3,4-thiadiazoles and an appropriate alpha-haloaryl/heteroaryl ketones. Further 5-bromo (III), 5-thiocyanato (IV), 5-gaunylhydrazone (V) derivatives were synthesized in order to study the effect of these substituents on biological
Andanappa K Gadad et al.
Bioorganic & medicinal chemistry, 16(1), 276-283 (2007-10-17)
A series of 2-trifluoromethyl/sulfonamido-5,6-diarylsubstituted imidazo[2,1-b]-1,3,4-thiadiazole derivatives 15a-j have been synthesized by the reaction of 2-amino-5-trifluoromethyl/sulfonamido-1,3,4-thiadiazoles 14a-b and appropriately substituted alpha-bromo-1,2-(p-substituted)diaryl-1-ethanones 13a-h. Structures of these compounds were established by IR, (1)H NMR, (13)C NMR, Mass, and HRMS data. The selected compounds
Bridgehead nitrogen heterocycles. II. Formation by reaction of. alpha.-amino nitrogen heterocyclic compounds with chlorothioformyl chloride.
Pilgram K and Skiles RD.
The Journal of Organic Chemistry, 38(8), 1575-1578 (1973)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service