All Photos(1)
About This Item
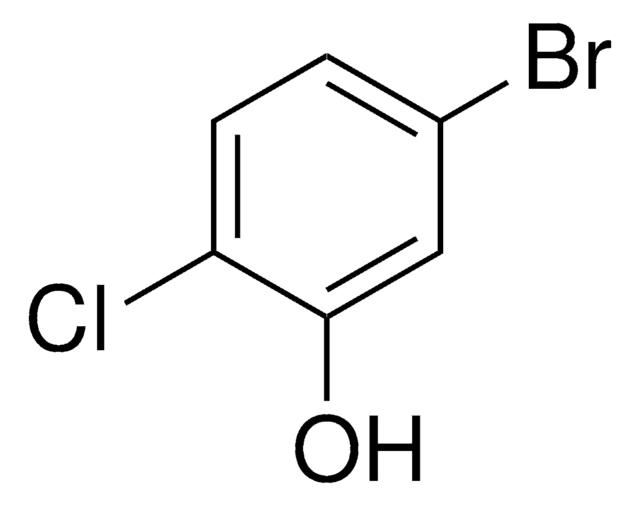
Linear Formula:
BrC6H3(Cl)OH
CAS Number:
Molecular Weight:
207.45
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
fibers
bp
232-235 °C (lit.)
mp
47-49 °C (lit.)
functional group
bromo
chloro
SMILES string
Oc1ccc(Br)cc1Cl
InChI
1S/C6H4BrClO/c7-4-1-2-6(9)5(8)3-4/h1-3,9H
InChI key
VIBJPUXLAKVICD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
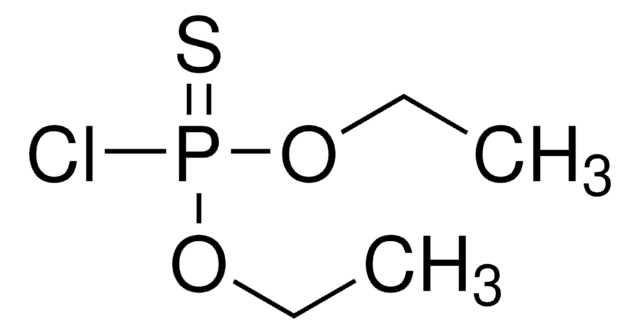
4-Bromo-2-chlorophenol is biologically inactive metabolite of profenofos, an organophosphorus pesticide. It undergoes enzyme-catalyzed copolymerization with phenols catalyzed by extracellular laccase of the fungus Rhizoctonia praticola.
Application
4-Bromo-2-chlorophenol was used as reagent during the synthesis of 7-arylbenzo
[b][1,4]oxazin derivatives.
[b][1,4]oxazin derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J J Sanchez Saez et al.
Food additives and contaminants, 8(5), 627-631 (1991-09-01)
An off-odour, described by the growers as similar to profenofos, occurred in melons in which this pesticide had been used in crop treatment. However, profenofos, O-(4-bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate, could not be detected in the melons using GC/MS although a
Mingbo Ma et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 54(1), 70-75 (2019-01-12)
Pesticides carried by cotton fiber are potential risk for production workers and consumers. Dissipation behaviour of a commonly used cotton pesticide profenofos in cotton fiber during growing period and scouring treatment was investigated. The results showed that profenofos in the
Solid-phase Synthesis of 7-Aryl-benzo [b][1, 4] oxazin-3 (4H)-one Derivatives on a BOMBA Resin Utilizing the Smiles Rearrangement.
Lee JM, et al.
Bull. Korean Chem. Soc., 30(6), 1325-1330 (2009)
Copolymerization of halogenated phenols and syringic acid.
Bollag J-M and Liu S-Y.
Pesticide Biochemistry and Physiology, 23(2), 261-272 (1985)
Sapna Yadav et al.
Environmental science and pollution research international, 24(3), 3074-3083 (2016-11-18)
In this study, the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method was applied for the analysis of the multiclass pesticide residues of 12 organochlorines (OCs), 9 organophosphates (OPs), 11 synthetic pyrethroids (SPs), 4 herbicides, 6 phthalates in raw
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service