164283
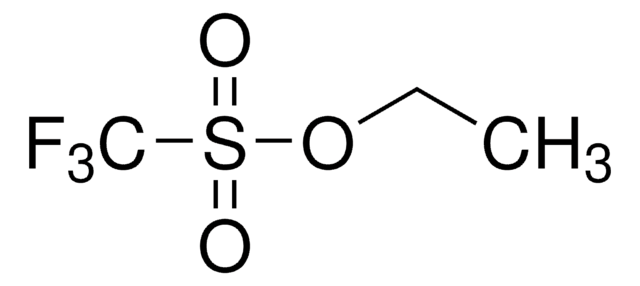
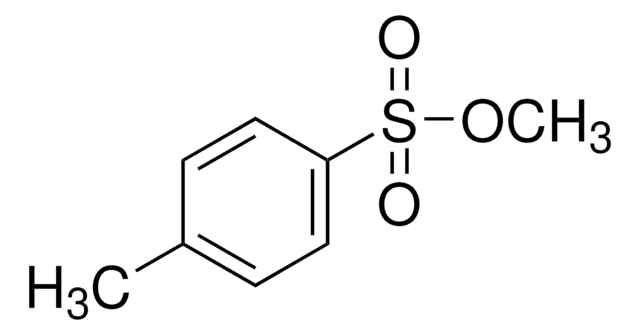
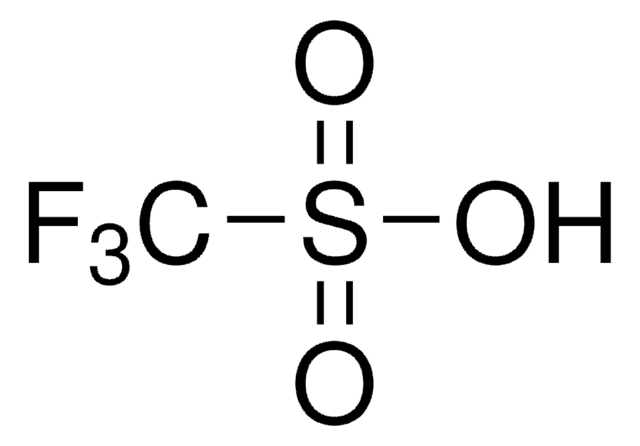
Methyl trifluoromethanesulfonate
≥98%
Synonym(s):
Methyl triflate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3SO2OCH3
CAS Number:
Molecular Weight:
164.10
Beilstein:
774772
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
liquid
refractive index
n20/D 1.326 (lit.)
bp
94-99 °C (lit.)
density
1.45 g/mL at 25 °C (lit.)
functional group
fluoro
triflate
storage temp.
2-8°C
SMILES string
COS(=O)(=O)C(F)(F)F
InChI
1S/C2H3F3O3S/c1-8-9(6,7)2(3,4)5/h1H3
InChI key
OIRDBPQYVWXNSJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl trifluoromethanesulfonate is a strong methylating reagent, commonly used for the pre-methylation of polysaccharides under mild basic conditions.
Application
Methyl trifluoromethanesulfonate can be used as a methylation reagent:
- In the determination of polysulfides, zerovalent sulfur in sulfide-rich water wells, and polysulfide species in electrolyte of a lithium–sulfur battery using chromatography-based techniques.
- In reactions with potassium enolates.
- For the conversion of amines to methyl ammonium triflates.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
100.4 °F - closed cup
Flash Point(C)
38 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ring Expansions of 2-Alkenylazetidinium Salts-a New Route to Pyrrolidines and Azepanes
Couty F, et al.
European Journal of Organic Chemistry, 2006(18), 4214-4223 (2006)
Oliver Schuster et al.
Inorganic chemistry, 45(20), 7997-7999 (2006-09-27)
Two cationic carbene complexes with no heteroatom in the ring containing the carbene carbon, trans-bromo(2-methyl-2,6-dihydroisoquinolin-6-ylidene)bis(triphenylphosphine)palladium(II) triflate (3) and trans-chloro(1,2-dimethyl-1,7-dihydroquinolin-7-ylidene)bis(triphenylphosphine)palladium(II) triflate (4), were synthesized by oxidative substitution of Pd(PPh3)4 with N-methylated 6-bromoisoquinolinium and 7-chloro-2-methylquinolinium cations, respectively. Compound 3 was also prepared
Comparison of [11C]methyl triflate and [11C]methyl iodide in the synthesis of PET radioligands such as [11C]beta-CIT and [11C]beta-CFT.
K Någren et al.
Nuclear medicine and biology, 22(8), 965-979 (1995-11-01)
J Passchier et al.
Synapse (New York, N.Y.), 64(7), 542-549 (2010-03-03)
The type-1 glycine transporter (GlyT1) is an important target for the development of new medications for schizophrenia. A specific and selective positron emission tomography (PET) GlyT1 ligand would facilitate drug development studies to determine whether a drug reaches this target
Yoshinori Takahashi et al.
Dalton transactions (Cambridge, England : 2003), (27)(27), 3546-3552 (2008-07-03)
Reaction of [Cp*Ir(micro-H)](2) (5) (Cp* = eta(5)-C(5)Me(5)) with bis(dimethylphosphino)methane (dmpm) gives a new neutral diiridium complex [(Cp*Ir)(2)(micro-dmpm)(micro-H)(2)] (3). Treatment of 3 with methyl triflate at -30 degrees C results in the formation of [(Cp*Ir)(H)(micro-dmpm)(micro-H)(Me)(IrCp*)][OTf] (6). Warming a solution of above
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service