158194
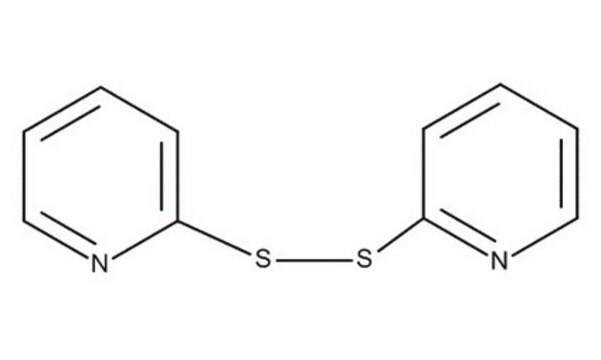
2,2′-Dithiobis(5-nitropyridine)
96%
Synonym(s):
Bis(5-nitro-2-pyridyl) disulfide, DTNP
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H6N4O4S2
CAS Number:
Molecular Weight:
310.31
Beilstein:
305413
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
155-157 °C (lit.)
functional group
disulfide
nitro
SMILES string
[O-][N+](=O)c1ccc(SSc2ccc(cn2)[N+]([O-])=O)nc1
InChI
1S/C10H6N4O4S2/c15-13(16)7-1-3-9(11-5-7)19-20-10-4-2-8(6-12-10)14(17)18/h1-6H
InChI key
ROUFCTKIILEETD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,2′-Dithiobis(5-nitropyridine) is an aromatic disulphide.
Application
2,2′-Dithiobis(5-nitropyridine) was employed:
- as cysteine-activating reagent to study the NMR of G protein-coupled receptors
- in the deprotection assays for protected selenocysteine-containing peptides
- for deprotecting p-methoxybenzyl groups and acetamidomethyl groups from the side-chains of cysteine and selenocysteine
- to remove the p-methoxybenzyl protecting group from cysteine and selenocysteine side-chains
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G J Stephens et al.
Pflugers Archiv : European journal of physiology, 431(3), 435-442 (1996-01-01)
The effects of cysteine-modifying reagents on the gating of rat cloned Kv1.4 channels expressed in HEK-293 cells were examined using the whole-cell patch-clamp technique. Cells transfected with Kv1.4 expressed a rapidly inactivating K+ current with a mid-point of activation of
A Li et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 18(17), 6740-6747 (1998-08-26)
Functional modifications of neuronal P/Q-type voltage-dependent Ca2+ channels expressed in Xenopus oocytes by oxidation were examined electrophysiologically. Oxidation by external H2O2 enhanced the whole-oocyte currents through the Ca2+ channels composed of the alpha1A, alpha2/delta, and beta3 subunits at negative voltages
Leah S Cohen et al.
Biopolymers, 102(1), 16-29 (2013-07-31)
Structural analysis by NMR of G protein-coupled receptors (GPCRs) has proven to be extremely challenging. To reduce the number of peaks in the NMR spectra by segmentally labeling a GPCR, we have developed a Guided Reconstitution method that includes the
P Sharma et al.
Analytical biochemistry, 189(2), 173-177 (1990-09-01)
A sensitive and simple method is described for the quantitative determination of free sulfhydryl (-SH) groups on polymer supports. The method includes the reaction of 4,4'-dimethoxytrityloxy-S-(2-thio-5-nitropyridyl)-2-mercapto ethane (DTNPME) with polymer-supported sulfhydryl groups. After removal of excess reagent through washing, a
Alayne L Schroll et al.
Journal of peptide science : an official publication of the European Peptide Society, 18(1), 1-9 (2011-11-16)
Of all the commercially available amino acid derivatives for solid phase peptide synthesis, none has a greater abundance of side-chain protection diversity than cysteine. The high reactivity of the cysteine thiol necessitates its attenuation during peptide construction. Moreover, the propensity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service