All Photos(1)
About This Item
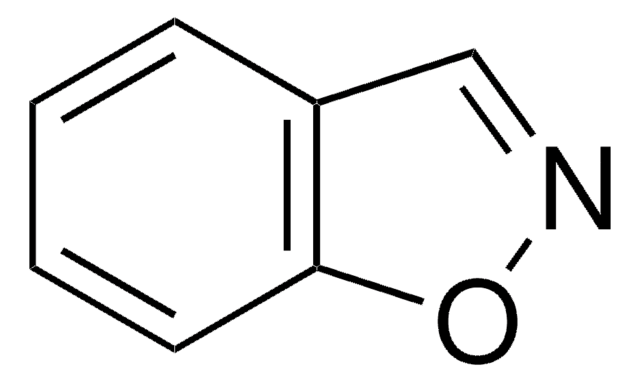
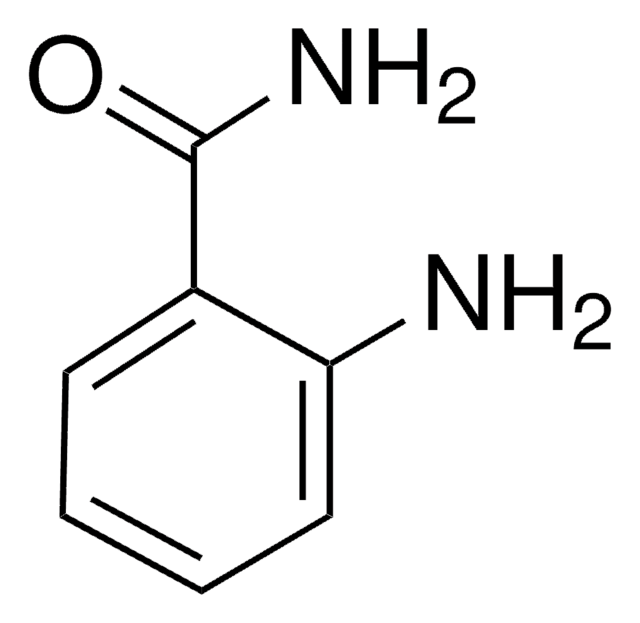
Empirical Formula (Hill Notation):
C7H5NO
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.584 (lit.)
bp
101-102 °C/15 mmHg (lit.)
density
1.183 g/mL at 25 °C (lit.)
SMILES string
c1ccc2nocc2c1
InChI
1S/C7H5NO/c1-2-4-7-6(3-1)5-9-8-7/h1-5H
InChI key
FZKCAHQKNJXICB-UHFFFAOYSA-N
General description
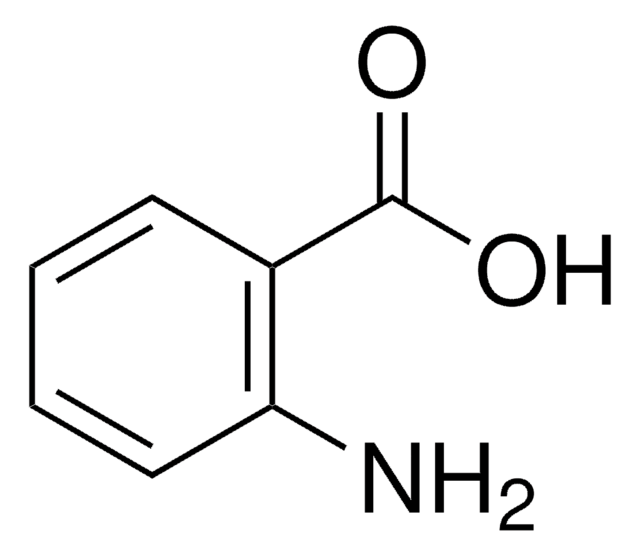
Anthranil undergoes thermal decomposition during single pulse shock-tube experiments to form aniline and cyclopentadiene carbonitrile. Surface-enhanced Raman spectrum of anthranil in activated silver colloid has been studied.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Assa Lifshitz et al.
The journal of physical chemistry. A, 110(27), 8248-8258 (2006-07-11)
The thermal decomposition of anthranil diluted in argon was studied behind reflected shock waves in a 2 in. i.d. pressurized driver single-pulse shock tube over the temperature range 825-1000 K and overall densities of approximately 3 x 10(-5) mol/cm(3). Two
Further exploration of stages in carcinogenesis.
V Armuth et al.
Carcinogenesis; a comprehensive survey, 7, 41-42 (1982-01-01)
Marna Pippel et al.
Bioorganic & medicinal chemistry letters, 19(22), 6373-6375 (2009-10-09)
A series of CCK2R-selective anthranilic amides is shown to derive CCK1R affinity via selective substitution of the amide side chain. Thus, extending the length of the original benzamide side chain by a single methylene unit imparts CCK1R affinity to the
Surface-enhanced Raman scattering and density functional theoretical study of anthranil adsorbed on colloidal silver particles.
Baia, M, et al.
The Journal of Physical Chemistry B, 108(45), 17491-17496 (2004)
Marna Pippel et al.
Bioorganic & medicinal chemistry letters, 19(22), 6376-6378 (2009-10-10)
In the previous article we demonstrated how certain CCK2R-selective anthranilic amides could be structurally modified to afford high-affinity, selective CCK1R activity. We now describe our efforts at modulating and optimizing the CCK1R and CCK2R affinities aimed at producing compounds with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service