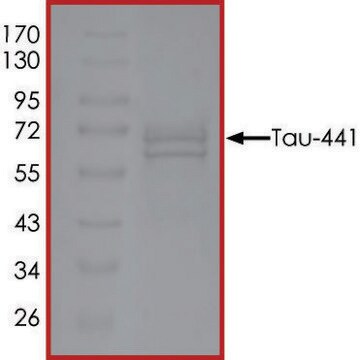
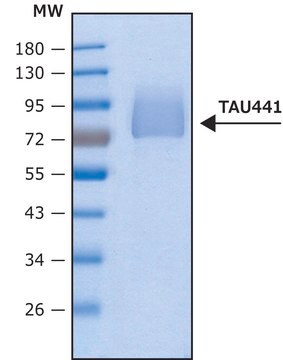
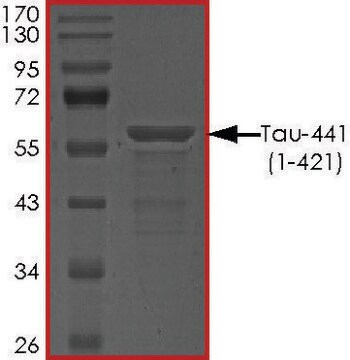
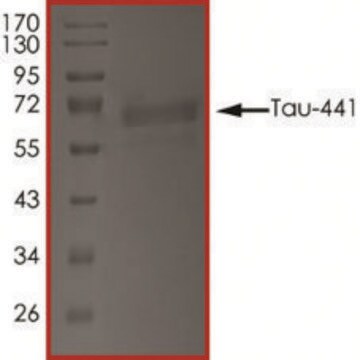
T9825
Tau-383 human
recombinant, expressed in E. coli, ≥90% (SDS-PAGE), lyophilized powder
Sinónimos:
MAPTL, MTBT1, TAU
About This Item
Productos recomendados
origen biológico
human
Nivel de calidad
recombinante
expressed in E. coli
Análisis
≥90% (SDS-PAGE)
formulario
lyophilized powder
mol peso
40 kDa
técnicas
western blot: suitable
idoneidad
suitable for Western blot
Nº de acceso UniProt
aplicaciones
cell analysis
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
Información sobre el gen
human ... MAPT(4137)
Descripción general
Tau-383 belongs to the neuronal microtubule-associated protein family and is found in the axons of the CNS (central nervous system). It is encoded by the MAPT (microtubule associated protein tau) gene on chromosome 17q21 and exists in the form of 6 isoforms produced by the alternative splicing of m-RNA transcripts of MAPT (microtubule associated protein tau).
Aplicación
Acciones bioquímicas o fisiológicas
Reconstitución
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico