SRP3324
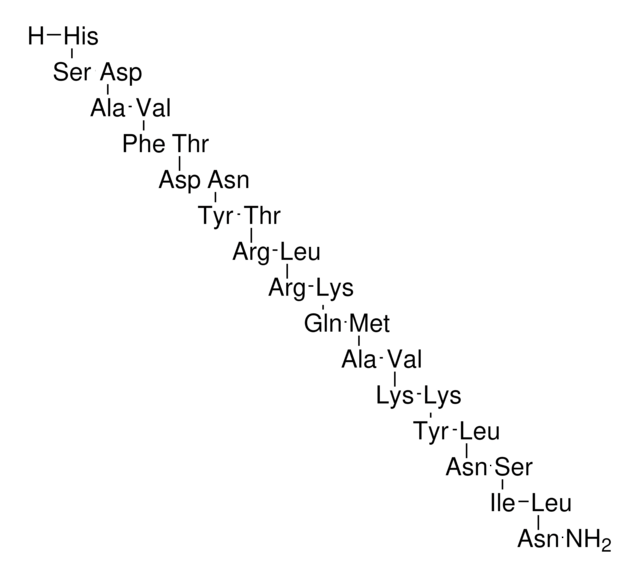
Thymosin β4 human
recombinant, expressed in E. coli, ≥95% (SDS-PAGE), ≥95% (HPLC)
Sinónimos:
Hematopoietic system regulatory peptide, Seraspenide, T-4
About This Item
Productos recomendados
origen biológico
human
recombinante
expressed in E. coli
Ensayo
≥95% (HPLC)
≥95% (SDS-PAGE)
Formulario
lyophilized
potencia
0.5-10 μg/mL
mol peso
5.2 kDa
envase
pkg of 100 μg
impurezas
endotoxin, tested
Nº de acceso UniProt
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
Información sobre el gen
human ... TMSB4X(7114)
Descripción general
Acciones bioquímicas o fisiológicas
Forma física
Reconstitución
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico