SML2011
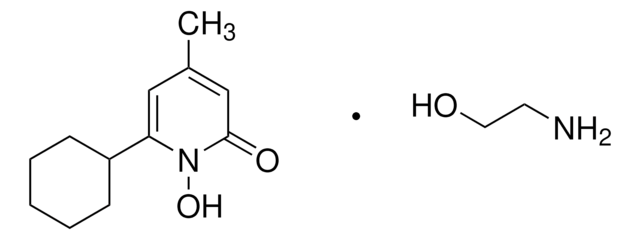
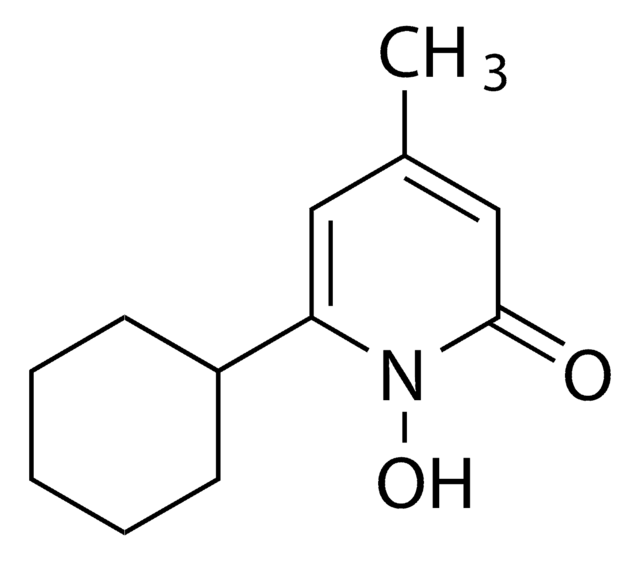
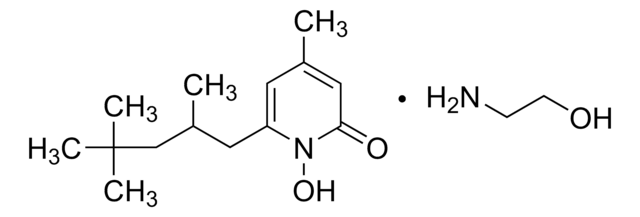
Ciclopirox
≥98% (HPLC)
Sinónimos:
Ciclopirox, 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone, HOE 296b, 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone
About This Item
Productos recomendados
Ensayo
≥98% (HPLC)
Formulario
powder
color
white to beige
solubilidad
DMSO: 2 mg/mL, clear
Condiciones de envío
ambient
temp. de almacenamiento
2-8°C
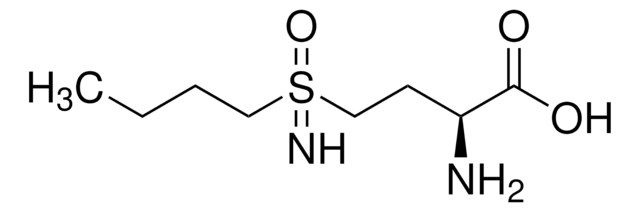
cadena SMILES
ON1C(C2CCCCC2)=CC(C)=CC1=O
InChI
1S/C12H17NO2/c1-9-7-11(13(15)12(14)8-9)10-5-3-2-4-6-10/h7-8,10,15H,2-6H2,1H3
Clave InChI
SCKYRAXSEDYPSA-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Acciones bioquímicas o fisiológicas
Palabra de señalización
Warning
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico