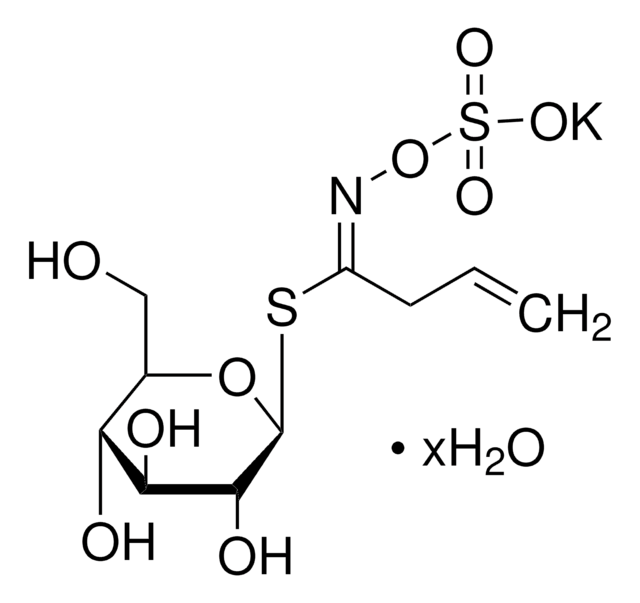
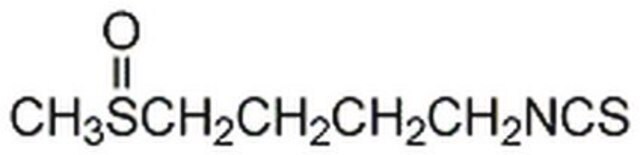S4441
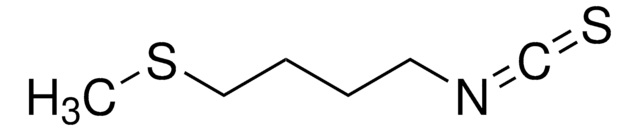
D,L-Sulforaphane
≥90% (HPLC), liquid, phase II detoxification enzymes inducer
Sinónimos:
1-Isothiocyanato-4-(methylsulfinyl)-butane
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H11NOS2
Número de CAS:
Peso molecular:
177.29
Número MDL:
Código UNSPSC:
12352200
ID de la sustancia en PubChem:
NACRES:
NA.77
Productos recomendados
Nombre del producto
DL-Sulforaphane, ≥90% (HPLC), synthetic, liquid
origen biológico
synthetic
Nivel de calidad
Ensayo
≥90% (HPLC)
Formulario
liquid
solubilidad
DMSO: soluble 40 mg/mL
H2O: insoluble
compatibilidad
for use with ABI 7500
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
cadena SMILES
CS(=O)CCCCN=C=S
InChI
1S/C6H11NOS2/c1-10(8)5-3-2-4-7-6-9/h2-5H2,1H3
Clave InChI
SUVMJBTUFCVSAD-UHFFFAOYSA-N
Aplicación
LNCaP prostate cancer cells were treated with DL-Sulforaphane to study the effect on androgen receptor. Effects on malathion toxicity was studied in rats by treating them with DL-Sulforaphane.
Acciones bioquímicas o fisiológicas
Selective inducer of phase II detoxification enzymes with anticarcinogenic properties. Organosulfur compound found in cruciferous vegetables, including broccoli.
Sulforaphane is an anti-cancer, anti-microbial and anti-diabetic compound found in cruciferous vegetables. It induces the production of detoxifying enzymes such as quinone reductase and glutathione S-transferase that cause xenobiotic transformation. Sulforaphane also increases the transcription of tumor suppressor proteins and inhibits histone deacetylases. It modulates inflammatory responses by suppressing the LPS-mediated expression of iNOS, COX-2, IL-1β and TNF-α.
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Equipo de protección personal
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
A Kenneth MacLeod et al.
British journal of cancer, 115(12), 1530-1539 (2016-11-09)
Although the nuclear factor-erythroid 2-related factor 2 (NRF2) pathway is one of the most frequently dysregulated in cancer, it is not clear whether mutational status is a good predictor of NRF2 activity. Here we utilise four members of the aldo-keto
Verena Jeschke et al.
Frontiers in plant science, 8, 1995-1995 (2017-12-07)
Multiple lepidopteran larvae feed successfully on plants containing glucosinolates despite the diverse array of toxic and deterrent breakdown products, such as isothiocyanates (ITCs), formed upon plant damage. While much is known about how specialist lepidopterans metabolize and tolerate glucosinolates, there
Carmela Fimognari et al.
Mutation research, 635(2-3), 90-104 (2006-12-01)
A number of natural compounds with inhibitory effects on tumorigenesis have been identified from our diet. Several studies have documented the cancer-preventive activity of a significant number of isothiocyanates (ITCs), the majority of which occur in plants, especially in Cruciferous
Masayoshi Yamaguchi et al.
International journal of molecular medicine, 38(6), 1940-1946 (2016-11-15)
β-caryophyllene, which is a constituent of many essential oils, has been known to be a selective agonist of the cannabinoid receptor type-2 and to exert cannabimimetic anti-inflammatory effects in animals. The effects of β-caryophyllene on macrophages have not been investigated
Systems Approach Reveals Nuclear Factor Erythroid 2-Related Factor 2/Protein Kinase R Crosstalk in Human Cutaneous Leishmaniasis.
Áislan de Carvalho Vivarini et al.
Frontiers in immunology, 8, 1127-1127 (2017-09-30)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico