L2626
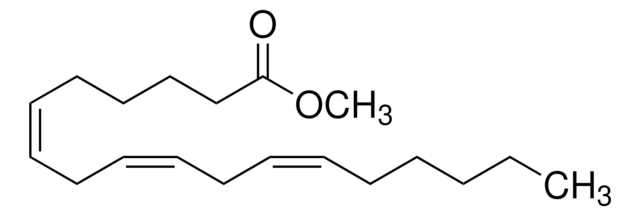
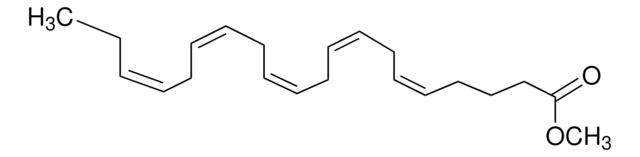
Methyl linolenate
≥99% (GC)
Sinónimos:
Linolenic acid methyl ester, Methyl cis,cis,cis-9,12,15-octadecatrienoate
About This Item
Productos recomendados
origen biológico
plant
Nivel de calidad
Análisis
≥99% (GC)
formulario
liquid
índice de refracción
n20/D 1.470 (lit.)
bp
182 °C/3 mmHg (lit.)
densidad
0.895 g/mL at 25 °C (lit.)
grupo funcional
ester
tipo de lípido
unsaturated FAs
Condiciones de envío
ambient
temp. de almacenamiento
−20°C
cadena SMILES
CC\C=C/C\C=C/C\C=C/CCCCCCCC(=O)OC
InChI
1S/C19H32O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h4-5,7-8,10-11H,3,6,9,12-18H2,1-2H3/b5-4-,8-7-,11-10-
Clave InChI
DVWSXZIHSUZZKJ-YSTUJMKBSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- Exogenous Jasmonic Acid Alleviates Blast Resistance Reduction Caused by LOX3 Knockout in Rice.: The research explores the role of jasmonic acid, associated with methyl linolenate, in enhancing blast resistance in rice. The findings show that external application of jasmonic acid can compensate for the resistance loss due to LOX3 knockout, providing insights into plant disease management strategies (Su et al., 2023).
- Effects of (13)C isotope-labeled allelochemicals on the growth of the invasive plant Alternanthera philoxeroides.: This study uses (13)C-labeled methyl linolenate to trace allelochemical effects on invasive plant species. The results indicate a significant impact on plant growth, suggesting potential applications in controlling invasive species (Hua et al., 2023).
Acciones bioquímicas o fisiológicas
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
235.4 °F - closed cup
Punto de inflamabilidad (°C)
113.0 °C - closed cup
Equipo de protección personal
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico