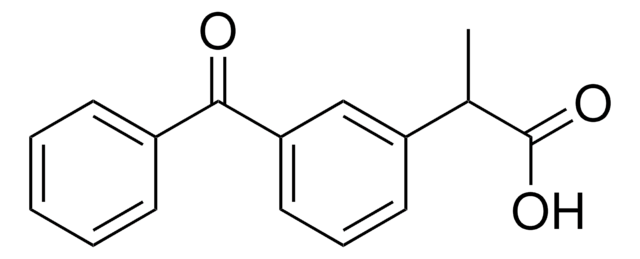
K1136
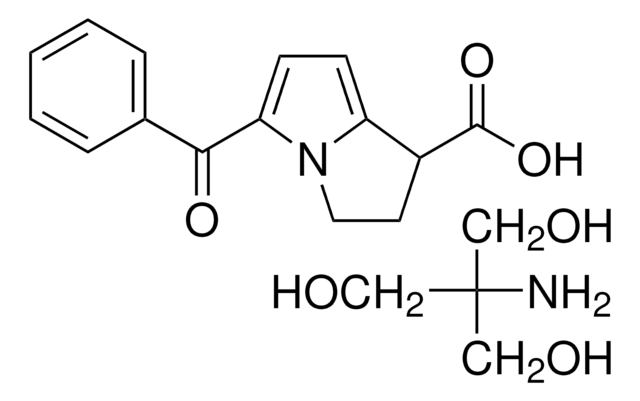
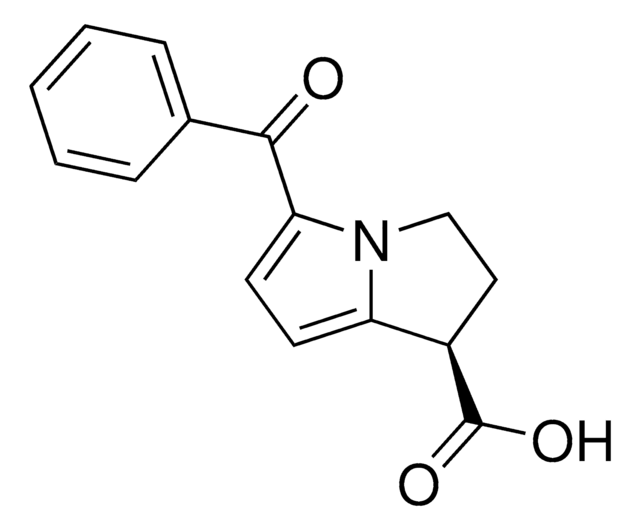
Ketorolac tris salt
≥99%, crystalline
Sinónimos:
(±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid tris salt, Toradol
About This Item
Productos recomendados
origen biológico
synthetic (organic)
Análisis
≥99%
formulario
crystalline
solubilidad
H2O: soluble 15 mg/mL, clear, colorless to faintly yellow (stable at least one month at −20 °C.)
temp. de almacenamiento
room temp
cadena SMILES
NC(CO)(CO)CO.OC(=O)C1CCn2c1ccc2C(=O)c3ccccc3
InChI
1S/C15H13NO3.C4H11NO3/c17-14(10-4-2-1-3-5-10)13-7-6-12-11(15(18)19)8-9-16(12)13;5-4(1-6,2-7)3-8/h1-7,11H,8-9H2,(H,18,19);6-8H,1-3,5H2
Clave InChI
BWHLPLXXIDYSNW-UHFFFAOYSA-N
Información sobre el gen
human ... PTGS1(5742) , PTGS2(5743)
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- as an intraperitoneal injection in mice to study the effect of ketorolac on expression of c-Fos (a human proto-oncogene) in ARC (arcuate nucleus of the hypothalamus) POMC (proopiomelanocortin) -EGFP (enhanced green fluorescent protein) neurons
- to treat mice in order to show that this treatment does not prevent IL-1β-mediated inhibition of Agouti-related protein (AgRP) secretion from murine hypothalamic explants
- as an analgesic medication to treat rats induced with acute inflammatory joint injury by injecting carrageenan into the ankle
Acciones bioquímicas o fisiológicas
Precaución
Aplicación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Chromatograms
application for HPLCNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico