G1774
Glucagon
≥95% (HPLC), powder, synthetic
About This Item
Productos recomendados
origen biológico
synthetic
Nivel de calidad
esterilidad
non-sterile
Ensayo
≥95% (HPLC)
Formulario
powder
técnicas
cell based assay: suitable
solubilidad
1% acetic acid: 1.00-1.04 mg/mL, clear, colorless
water: 1.00-1.04 mg/mL, clear, colorless
idoneidad
suitable for molecular biology
Nº de acceso UniProt
Condiciones de envío
ambient
temp. de almacenamiento
−20°C
cadena SMILES
Cl[H].[H]N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](C(C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc4ccc(O)cc4)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc5ccccc5)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc6c[nH]c7ccccc67)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)O)C(O)=O
InChI
1S/C153H225N43O49S.ClH/c1-72(2)52-97(133(226)176-96(47-51-246-11)132(225)184-104(60-115(159)209)143(236)196-123(78(10)203)151(244)245)179-137(230)103(58-83-64-167-89-29-19-18-28-87(83)89)183-131(224)95(43-46-114(158)208)177-148(241)120(74(5)6)194-141(234)101(54-79-24-14-12-15-25-79)182-138(231)105(61-117(211)212)185-130(223)94(42-45-113(157)207)171-124(217)75(7)170-127(220)91(31-22-49-165-152(160)161)172-128(221)92(32-23-50-166-153(162)163)174-146(239)110(69-199)191-140(233)107(63-119(215)216)186-134(227)98(53-73(3)4)178-135(228)99(56-81-33-37-85(204)38-34-81)180-129(222)90(30-20-21-48-154)173-145(238)109(68-198)190-136(229)100(57-82-35-39-86(205)40-36-82)181-139(232)106(62-118(213)214)187-147(240)111(70-200)192-150(243)122(77(9)202)195-142(235)102(55-80-26-16-13-17-27-80)188-149(242)121(76(8)201)193-116(210)66-168-126(219)93(41-44-112(156)206)175-144(237)108(67-197)189-125(218)88(155)59-84-65-164-71-169-84;/h12-19,24-29,33-40,64-65,71-78,88,90-111,120-123,167,197-205H,20-23,30-32,41-63,66-70,154-155H2,1-11H3,(H2,156,206)(H2,157,207)(H2,158,208)(H2,159,209)(H,164,169)(H,168,219)(H,170,220)(H,171,217)(H,172,221)(H,173,238)(H,174,239)(H,175,237)(H,176,226)(H,177,241)(H,178,228)(H,179,230)(H,180,222)(H,181,232)(H,182,231)(H,183,224)(H,184,225)(H,185,223)(H,186,227)(H,187,240)(H,188,242)(H,189,218)(H,190,229)(H,191,233)(H,192,243)(H,193,210)(H,194,234)(H,195,235)(H,196,236)(H,211,212)(H,213,214)(H,215,216)(H,244,245)(H4,160,161,165)(H4,162,163,166);1H/t75-,76+,77+,78+,88-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-;/m0./s1
Clave InChI
RKGLLHCSSVJTAN-YYICOITRSA-N
Información sobre el gen
human ... GCG(2641)
¿Está buscando productos similares? Visita Guía de comparación de productos
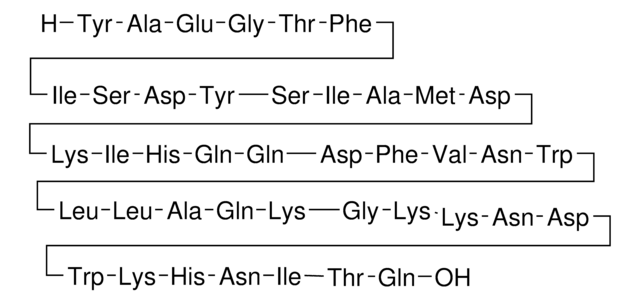
Amino Acid Sequence
Descripción general
Aplicación
- used as a component of hormone stock solution for preserving full biological activity of heart tissues obtained from male Sprague-Dawley rats
- used as an infusion in phases II and III fasting king penguins to study the various lipolytic, metabolic, and hormonal responses
- used for the stimulation of PGC-1α expression in hepatocytes
- used to induce the expression of methionine adenosyltransferase α1 (MAT1A), which is involved in the regulation of hepatic levels of S-adenosylmethionine and the adaptive response to fasting
- used to study its effect on stimulation of gluconeogenesis through hepatic lipolysis, mediated by inositol trisphosphate receptor 1 (INSP3R1)
- intraperitoneally injected in mice to assess glucagon-induced Sam68 subcellular localization in vivo
Acciones bioquímicas o fisiológicas
Componentes
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico


![[Arg8]-Vasopressin solution Grade VI (synthetic), ~100 IU/mL in 0.9% NaCl](/deepweb/assets/sigmaaldrich/product/structures/326/242/dede8c26-cf73-4a28-a5d9-1d57c673cf0e/640/dede8c26-cf73-4a28-a5d9-1d57c673cf0e.png)