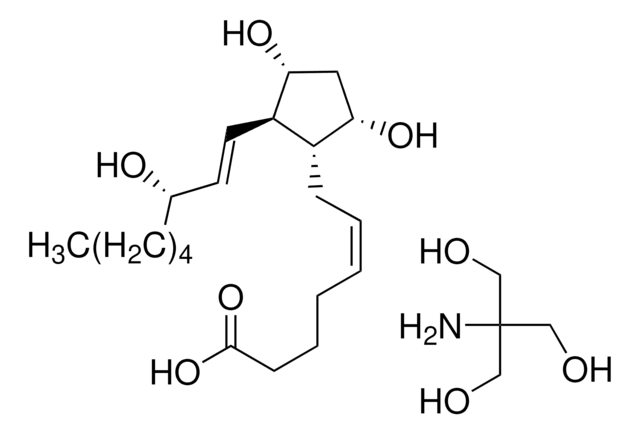
D8174
9,11-Dideoxy-11α,9α-epoxymethanoprostaglandin F2α
≥98% (HPLC), solution, thromboxane A2 agonist
Sinónimos:
U-46619, U46619
About This Item
Productos recomendados
Nombre del producto
9,11-Dideoxy-11α,9α-epoxymethanoprostaglandin F2α, solution, 10 mg/mL in methyl acetate
Formulario
solution
Nivel de calidad
concentración
10 mg/mL in methyl acetate
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
cadena SMILES
CCCCC[C@H](O)\C=C\[C@H]1C2CC(CO2)[C@@H]1C\C=C/CCCC(O)=O
InChI
1S/C21H34O4/c1-2-3-6-9-17(22)12-13-19-18(16-14-20(19)25-15-16)10-7-4-5-8-11-21(23)24/h4,7,12-13,16-20,22H,2-3,5-6,8-11,14-15H2,1H3,(H,23,24)/b7-4-,13-12+/t16?,17-,18-,19+,20?/m0/s1
Clave InChI
LQANGKSBLPMBTJ-REGKDVDGSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- to induce aortic smooth muscle (SM) contraction in mice deficient in myosin light chain 9 (Myl9) gene
- to induce contraction as part of vascular reactivity experiments using mice aorta
- as a thromboxane/prostaglandin agonist to study the effect of dithiothreitol (DTT) on mice arterial vessel viability
Acciones bioquímicas o fisiológicas
Características y beneficios
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Órganos de actuación
Central nervous system
Riesgos supl.
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
14.0 °F
Punto de inflamabilidad (°C)
-10 °C
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico