D4943
Dipeptidyl Peptidase IV human
recombinant, expressed in baculovirus infected Sf9 cells, pkg of ≥1.0 units/vial, ≥10 units/mg protein
Sinónimos:
CD26, DPPIV, Dipeptidyl aminopeptidase IV, Glycoprotein GP110
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Número MDL:
Código UNSPSC:
12352204
NACRES:
NA.54
Productos recomendados
recombinante
expressed in baculovirus infected Sf9 cells
Formulario
solution
actividad específica
≥10 units/mg protein
mol peso
105 kDa
envase
pkg of ≥1.0 units/vial
Nº de acceso UniProt
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
Información sobre el gen
human ... DPP4(1803)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
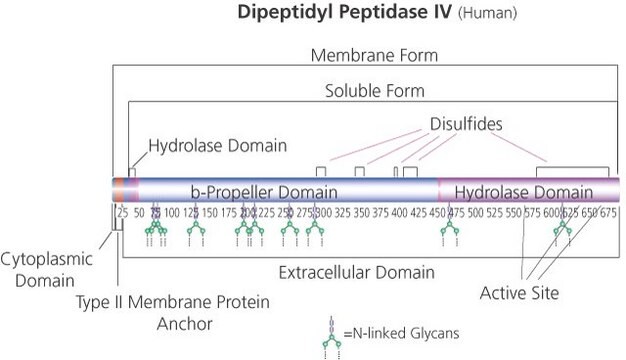
C-terminal histidine-tagged. Soluble form (residues 29-766) MW 105 kDa
Aplicación
Human dipeptidyl peptidase IV has been used to study interactive hemodynamic effects of its inhibition and angiotensin-converting enzyme inhibition in humans. Human dipeptidyl peptidase IV has also been used in a study that informed the understanding of Hymenoptera venom allergies.
The enzyme from Sigma has been used to study the LC-MS (liquid chromatography-mass spectrometry) based assay method for DPP-IV inhibitor screening and substrate discovery.
Acciones bioquímicas o fisiológicas
DPPIV has a post-proline dipeptidyl aminopeptidase activity that hydrolyzes N-terminal dipeptides from the unsubstituted N-terminus of peptides with the sequence of X-Pro-Z and X-Ala-Z. The optimum pH is found to be 7.4-8.7. DPPIV is involved in the regulation of several important physiological processes such as immune functions, inflammation, CNS, endocrine functions, bone marrow mobilization, cancer growth, cell adhesion, glucose hemostasis and sepsis/severe infection.
DPPIV has a post-proline dipeptidyl aminopeptidase activity that hydrolyzes N-terminal dipeptides from the unsubstituted N-terminus of peptides with the sequence of X-Pro-Z and X-Ala-Z. Where X is a nonspecific residue at the N terminus and Z cannot be proline or hydroxyproline.
Native DPPIV is a ubiquitous type II transmembrane glycoprotein and a serine protease of the S9 prolyl-oligopeptidase family. In vivo, it is synthesized with a signal peptide, which functions as the membrane anchoring domain. There is an 88% sequence homology between the human and porcine kidney enzymes. Both exist as homodimers with a subunit molecular weight of ~30 kDa. The high mannose 100 kDa DPPIV precursor is processed in the Golgi to yield a 124 kDa heavily N-and O-linked mature glycoprotein. It is then sorted to the apical membrane through the concerted action of both N- and O-linked glycans and its association with lipid microdomains. The porcine enzyme contains 18.3% carbohydrates, which the glycan composition is 0.9% fucose, 3.4% mannose, 5.1% galactose, 8.2% glucosamine, and 0.7% sialic acid. DPPIV is highly expressed on endothelial cells, epithelial cells, and lymphocytes. It is also present in plasma in its soluble form.
Definición de unidad
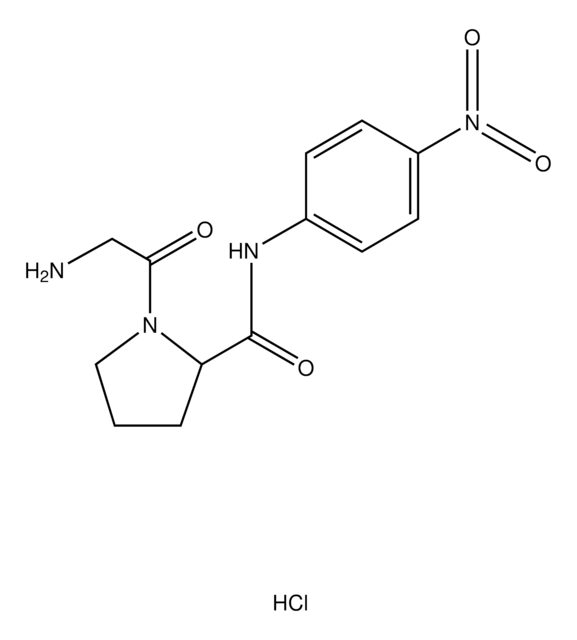
One unit will produce 1.0 μmole of p-nitroaniline from Gly-L-Pro p-nitroanilide per min in 100 mM Tris-HCl at pH 7.6 at 37 °C.
Forma física
Supplied as a solution in 10 mM Tris-HCl, pH 7.6, 200 mM NaCl, 1 mM EDTA and 10% glycerol.
Otras notas
View more information on Dipeptidyl Peptidase IV at www.sigma-aldrich.com/enzymeexplorer.
Inhibidor
Referencia del producto
Descripción
Precios
sustrato
Referencia del producto
Descripción
Precios
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
LC-MS based assay method for DPP-IV inhibitor screening and substrate discovery.
Liu, J., Cheng, X., & Fu, L.
Analytical Methods : Advancing Methods and Applications, 4(6), 1797-1805 (2012)
Ryugen Takahashi et al.
Frontiers in oncology, 11, 714527-714527 (2021-09-08)
Radical resection is the only curative treatment for pancreatic cancer, which is a life-threatening disease. However, it is often not easy to accurately identify the extent of the tumor before and during surgery. Here we describe the development of a
Mark D Gorrell
Clinical science (London, England : 1979), 108(4), 277-292 (2004-12-09)
DP (dipeptidyl peptidase) IV is the archetypal member of its six-member gene family. Four members of this family, DPIV, FAP (fibroblast activation protein), DP8 and DP9, have a rare substrate specificity, hydrolysis of a prolyl bond two residues from the
Petr Busek et al.
The international journal of biochemistry & cell biology, 36(3), 408-421 (2003-12-23)
Post-translational modification of proteins is an important regulatory event. Numerous biologically active peptides that play an essential role in cancerogenesis contain an evolutionary conserved proline residue as a proteolytic-processing regulatory element. Proline-specific proteases could therefore be viewed as important "check-points".
Ivan O Maslov et al.
Pharmaceuticals (Basel, Switzerland), 15(3) (2022-03-27)
Compounds that contain (R)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid substituted with bicyclic amino moiety (2-aza-bicyclo[2.2.1]heptane) were designed using molecular modelling methods, synthesised, and found to be potent DPP-4 (dipeptidyl peptidase-4) inhibitors. Compound 12a (IC50 = 16.8 ± 2.2 nM), named neogliptin, is a more
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico