B6804
Monoclonal Anti-Bacterial Alkaline Phosphatase (BAP, PhoA) antibody produced in mouse
clone BAP-77, ascites fluid
Sinónimos:
Monoclonal Anti-Phosphatase, Alkaline, bacterial
About This Item
Productos recomendados
origen biológico
mouse
Nivel de calidad
conjugado
alkaline phosphatase conjugate
forma del anticuerpo
ascites fluid
tipo de anticuerpo
primary antibodies
clon
BAP-77, monoclonal
mol peso
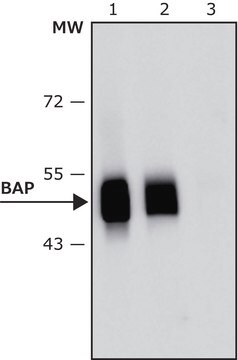
antigen ~50 kDa
reactividad de especies
bacteria
técnicas
indirect ELISA: suitable
western blot: 1:20,000 using purified Escherichia coli BAP
isotipo
IgG1
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
modificación del objetivo postraduccional
unmodified
Descripción general
Inmunógeno
Aplicación
Acciones bioquímicas o fisiológicas
Cláusula de descargo de responsabilidad
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico