A8001
Aconitine
≥95% (HPLC), crystalline
Sinónimos:
Acetylbenzoylaconine
About This Item
Productos recomendados
Análisis
≥95% (HPLC)
formulario
crystalline
color
white
solubilidad
H2O: 0.3 mg/mL
ethanol: 35 mg/mL
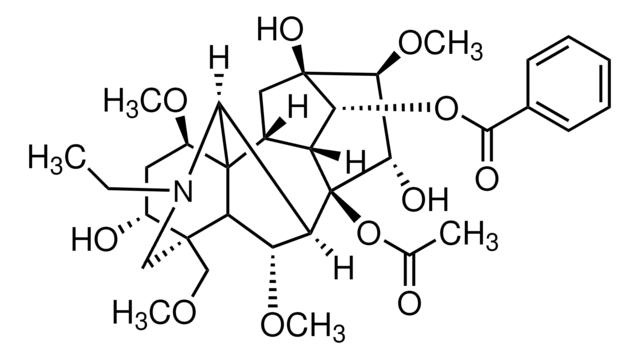
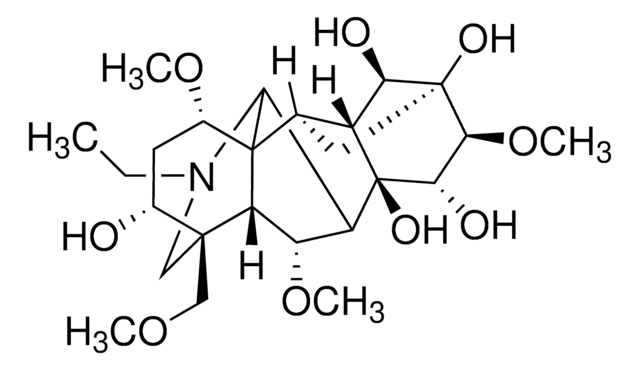
cadena SMILES
CCN1C[C@]2(COC)[C@H](O)C[C@@H](OC)C34C5C[C@]6(O)[C@@H](OC)[C@H](O)[C@@](OC(C)=O)(C5[C@H]6OC(=O)c7ccccc7)C([C@H](OC)C23)C14
InChI
1S/C34H47NO11/c1-7-35-15-31(16-41-3)20(37)13-21(42-4)33-19-14-32(40)28(45-30(39)18-11-9-8-10-12-18)22(19)34(46-17(2)36,27(38)29(32)44-6)23(26(33)35)24(43-5)25(31)33/h8-12,19-29,37-38,40H,7,13-16H2,1-6H3/t19-,20-,21-,22-,23+,24+,25?,26?,27+,28-,29+,31+,32-,33?,34-/m1/s1
Clave InChI
XFSBVAOIAHNAPC-VBUFWTEXSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
- to study its cardiotoxic effects along the pericardium meridian (PM) on cardiac rhythm in rabbits
- as a standard in high-performance thin layer chromatography (HPTLC) fingerprinting method
- in the aconitine-based lipo-alkaloids semi-synthesis
Acciones bioquímicas o fisiológicas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 1 Oral - Acute Tox. 2 Inhalation
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico