A5880
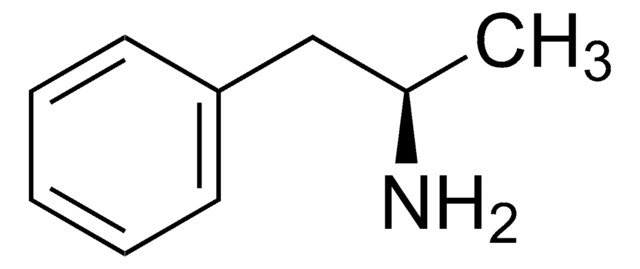
D-Amphetamine hemisulfate salt
Sinónimos:
(+)-α-Methylphenethylamine hemisulfate salt, Dextroamphetamine hemisulfate salt
About This Item
Productos recomendados
formulario
powder
control farmacológico
USDEA Schedule II; Home Office Schedule 2; stupéfiant (France); kontrollierte Droge in Deutschland; regulated under CDSA - not available from Sigma-Aldrich Canada
solubilidad
H2O: soluble
ethanol: soluble
aplicaciones
forensics and toxicology
cadena SMILES
OS(O)(=O)=O.CC(N)Cc1ccccc1.CC(N)Cc2ccccc2
InChI
1S/2C9H13N.H2O4S/c2*1-8(10)7-9-5-3-2-4-6-9;1-5(2,3)4/h2*2-6,8H,7,10H2,1H3;(H2,1,2,3,4)/t2*8-;/m00./s1
Clave InChI
PYHRZPFZZDCOPH-QXGOIDDHSA-N
Información sobre el gen
human ... SLC18A2(6571)
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Acciones bioquímicas o fisiológicas
Aplicación
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chromatograms
suitable for GCsuitable for GCapplication for HPLCsuitable for GCNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico