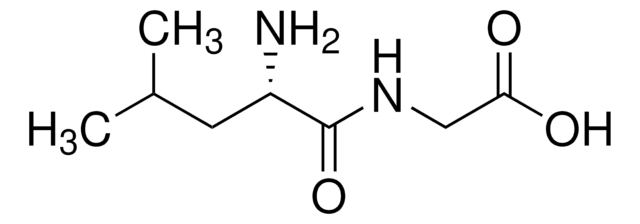
A3128
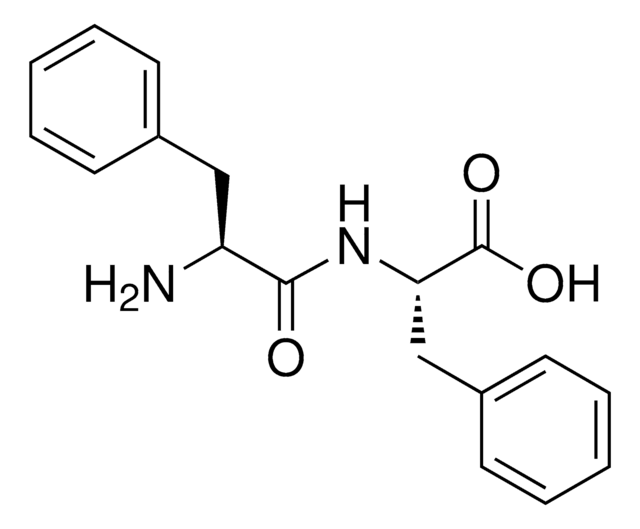
Ala-Phe
≥98% (TLC)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C12H16N2O3
Número de CAS:
Peso molecular:
236.27
Número MDL:
Código UNSPSC:
12352202
ID de la sustancia en PubChem:
NACRES:
NA.26
Productos recomendados
Nombre del producto
Ala-Phe,
Ensayo
≥98% (TLC)
Nivel de calidad
Formulario
powder
color
white to off-white
temp. de almacenamiento
−20°C
cadena SMILES
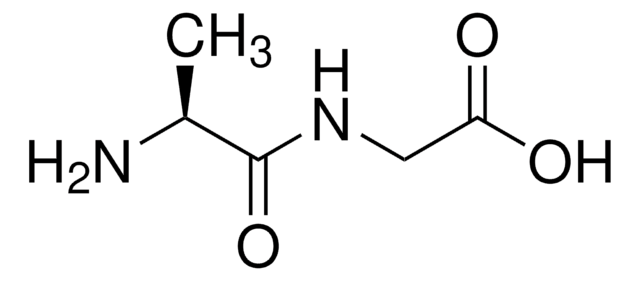
C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI
1S/C12H16N2O3/c1-8(13)11(15)14-10(12(16)17)7-9-5-3-2-4-6-9/h2-6,8,10H,7,13H2,1H3,(H,14,15)(H,16,17)/t8-,10-/m0/s1
Clave InChI
OMNVYXHOSHNURL-WPRPVWTQSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Amino Acid Sequence
Ala-Phe
Acciones bioquímicas o fisiológicas
Alanyl dipeptides such as ala-leu, ala-lys, ala-gly, ala-pro, ala-tyr and ala-phe may be used in physicochemical studies or to evaluate dipeptide separation technologies. Alanyl dipeptides may also be used for studying cell uptake mechanisms, dipeptide metabolism or cell growth supplementation benefits.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Yasuhito Shomura et al.
Protein science : a publication of the Protein Society, 21(5), 707-716 (2012-03-13)
BacD is an ATP-dependent dipeptide ligase responsible for the biosynthesis of L-alanyl-L-anticapsin, a precursor of an antibiotic produced by Bacillus spp. In contrast to the well-studied and phylogenetically related D-alanine: D-alanine ligase (Ddl), BacD synthesizes dipeptides using L-amino acids as
Elizabeth A Girnys et al.
Chemical biology & drug design, 75(1), 35-39 (2009-12-04)
Myocardial ischemia and other acute coronary syndromes are leading causes of death worldwide, and often result from a thrombus that blocks an atherosclerotic coronary artery. A key enzyme in thrombus formation is the serine protease thrombin, which is responsible for
Tomasz Pawlak et al.
The journal of physical chemistry. B, 116(6), 1974-1983 (2012-01-14)
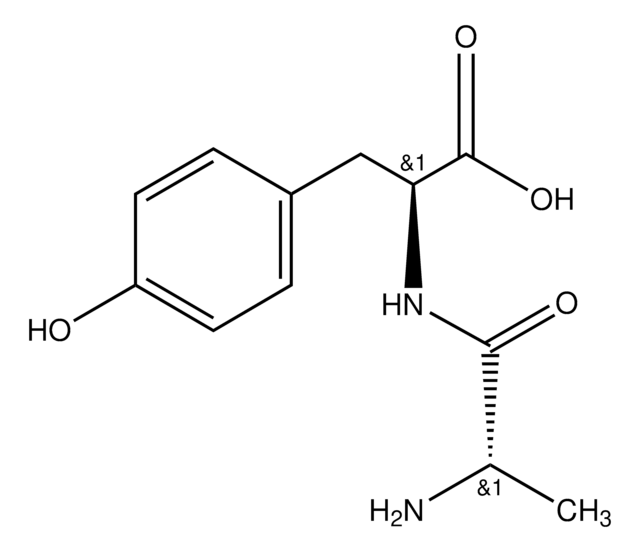
DFT methods were employed to compute the (13)C NMR chemical shift tensor (CST) parameters for crystals of YAF peptides (Tyr-Ala-Phe) with different stereochemistry for the Ala residue. Tyr-D-Ala-Phe 1 crystallizes in the C2 space group while Tyr-L-Ala-Phe crystallizes in either
J Li et al.
Electrophoresis, 20(1), 171-179 (1999-03-05)
The separation of stereoisomers, particularly enantiomers, is important when their physiological activity differs. We have resolved the four stereoisomers each of alanylphenylalanine (Ala-Phe) and of leucylphenylalanine (Leu-Phe) by capillary electrophoresis using beta-cyclodextrin as a buffer additive and urea to enhance
Semra Kocabiyik et al.
Protein expression and purification, 73(2), 223-230 (2010-05-13)
In this study we describe, the construction of a co-expression vector allowing simultaneous production of Thermoplasma volcanium 20S proteasome alpha- and beta-subunits in Escherichia coli. This heterologous expression system provided high level production of fully active 20S proteasome that can
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico