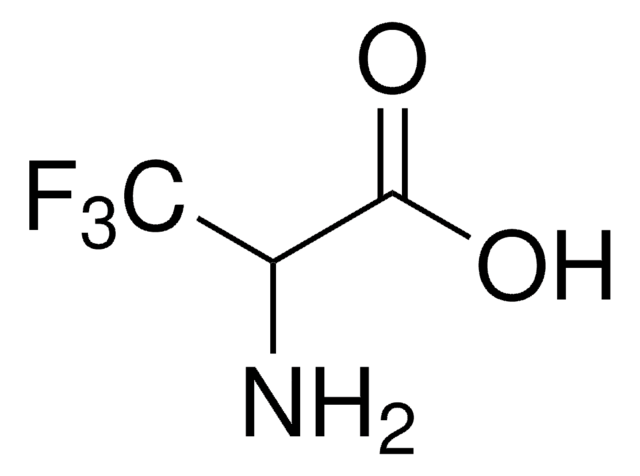
47568
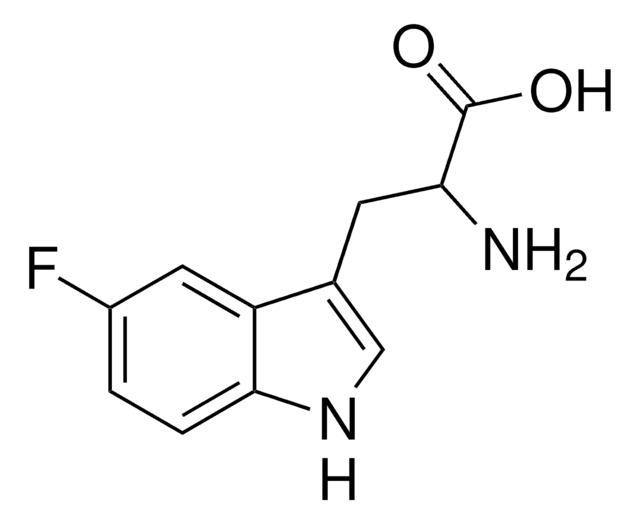
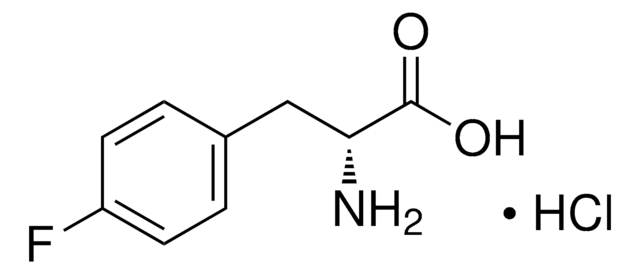
5-Fluoro-L-tryptophan
≥98.0% (HPLC)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C11H11FN2O2
Número de CAS:
Peso molecular:
222.22
Beilstein:
5052680
Número MDL:
Código UNSPSC:
12352202
eCl@ss:
32160406
ID de la sustancia en PubChem:
NACRES:
NA.77
Productos recomendados
Ensayo
≥98.0% (HPLC)
Formulario
powder
pureza óptica
enantiomeric ratio: ≥99.5:0.5 (HPLC)
mp
270-280 °C
temp. de almacenamiento
2-8°C
cadena SMILES
N[C@@H](Cc1c[nH]c2ccc(F)cc12)C(O)=O
InChI
1S/C11H11FN2O2/c12-7-1-2-10-8(4-7)6(5-14-10)3-9(13)11(15)16/h1-2,4-5,9,14H,3,13H2,(H,15,16)/t9-/m0/s1
Clave InChI
INPQIVHQSQUEAJ-VIFPVBQESA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
Exogenous 5-fluoro-Trp is incorporated into proteins in normal protein synthesis. Since 19F is a useful reporter group, this provides a method for studying enzyme mechanisms by NMR.
Acciones bioquímicas o fisiológicas
5-Fluoro-Trp is nonspecifically cytotoxic. It is believed this is due to malfunctioning enzymes that have had replacements of Trp residues by 5-fluoro-Trp. However, at least one case is known where 5-fluoro-Trp substitution leads to significantly greater catalytic activity.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
G S Rule et al.
Biochemistry, 26(2), 549-556 (1987-01-27)
In this study we demonstrate the potential of combining fluorine-19 nuclear magnetic resonance (NMR) spectroscopy with molecular genetics. We are using the membrane-bound enzyme D-lactate dehydrogenase of Escherichia coli as a model system to characterize interactions between proteins and lipids.
Dereje Abate Negatu et al.
mBio, 10(2) (2019-03-28)
Indole propionic acid (IPA), produced by the gut microbiota, is active against Mycobacterium tuberculosisin vitro and in vivo However, its mechanism of action is unknown. IPA is the deamination product of tryptophan (Trp) and thus a close structural analog of
E W Miles et al.
Biochemistry, 25(15), 4240-4249 (1986-07-29)
We are exploring the active site and the mechanism of the pyridoxal phosphate dependent reactions of the bacterial tryptophan synthase alpha 2 beta 2 complex by use of substrate analogues and of reaction intermediate analogues. Fluorine-19 nuclear magnetic resonance studies
Warintra Pitsawong et al.
eLife, 7 (2018-06-15)
Protein kinases are major drug targets, but the development of highly-selective inhibitors has been challenging due to the similarity of their active sites. The observation of distinct structural states of the fully-conserved Asp-Phe-Gly (DFG) loop has put the concept of
S Rozovsky et al.
Journal of molecular biology, 310(1), 271-280 (2001-06-23)
Product release is partially rate determining in the isomerization reaction catalyzed by Triosephosphate Isomerase, the conversion of dihydroxyacetone phosphate to D-glyceraldehyde 3-phosphate, probably because an active-site loop movement is necessary to free the product from confinement in the active-site. The
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico