P7754
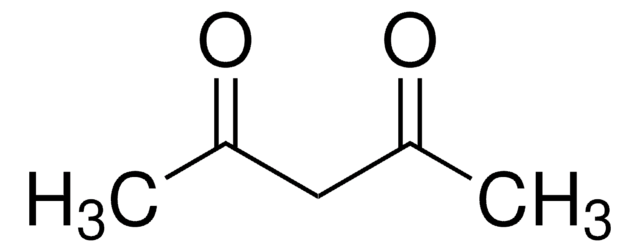
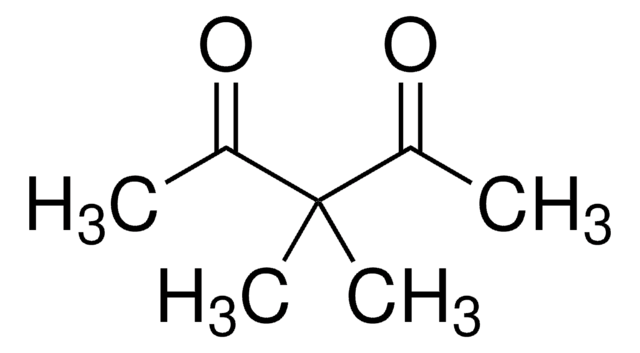
Acetilacetona
ReagentPlus®, ≥99%
Sinónimos:
2,4-Pentanodiona
About This Item
Productos recomendados
densidad de vapor
3.5 (vs air)
Nivel de calidad
presión de vapor
6 mmHg ( 20 °C)
Línea del producto
ReagentPlus®
Análisis
≥99%
formulario
liquid
temp. de autoignición
662 °F
lim. expl.
11.4 %
índice de refracción
n20/D 1.452 (lit.)
pH
6 (20 °C, 200 g/L)
bp
140.4 °C (lit.)
mp
−23 °C (lit.)
densidad
0.975 g/mL at 25 °C (lit.)
cadena SMILES
CC(=O)CC(C)=O
InChI
1S/C5H8O2/c1-4(6)3-5(2)7/h3H2,1-2H3
Clave InChI
YRKCREAYFQTBPV-UHFFFAOYSA-N
Información sobre el gen
human ... ACHE(43) , BCHE(590) , CES1(1066)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- A multifunctional ligand in the synthesis and feasible functionalization of gold nanoparticles (AuNPs).
- A reactant to synthesize 9,10-dihydroacridines by reacting with methyl acetoacetate and Morita-Baylis-Hillman acetates.
- A reagent in the synthesis of ZrO2(zirconium dioxide) via hydrolysis of Zr(OC3H7n)4. Acetylacetone controls the hydrolysis and condensation rates of alkoxides and thus, the nucleation and growth rates of oxides.
Envase
Información legal
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
95.0 °F - closed cup
Punto de inflamabilidad (°C)
35 °C - closed cup
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico