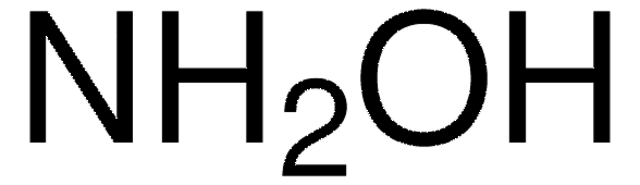159417
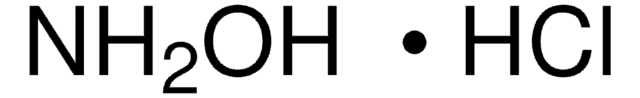
Hydroxylamine hydrochloride
ReagentPlus®, 99%
Sinónimos:
Hydroxylammonium chloride
About This Item
Productos recomendados
grado
reagent
Nivel de calidad
presión de vapor
0.001 hPa ( 50 °C)
Línea del producto
ReagentPlus®
Ensayo
99%
Formulario
crystalline
técnicas
inhibition assay: suitable
pH
2.5-3.5 (20 °C, 50 g/L)
mp
155-157 °C (dec.) (lit.)
densidad
1.67 g/mL at 25 °C (lit.)
cadena SMILES
Cl.NO
InChI
1S/ClH.H3NO/c;1-2/h1H;2H,1H2
Clave InChI
WTDHULULXKLSOZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- in the synthesis of primary amides from aldehydes in the presence of cesium carbonate (Cs2CO3) as a catalyst.
- in the conversion of alicyclic /aliphatic carbonyl compounds and the aromatic aldehydes into corresponding oximes.
- in the one-pot synthesis of nitriles from aldehydes in the presence of sodium sulfate (anhyd) and sodium bicarbonate catalysts.
- It can also be used as a reducing agent in the preparation of single-layer reduced graphene oxide (RGO) sheets and films.
Acciones bioquímicas o fisiológicas
Información legal
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Órganos de actuación
spleen
Código de clase de almacenamiento
4.1A - Other explosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico