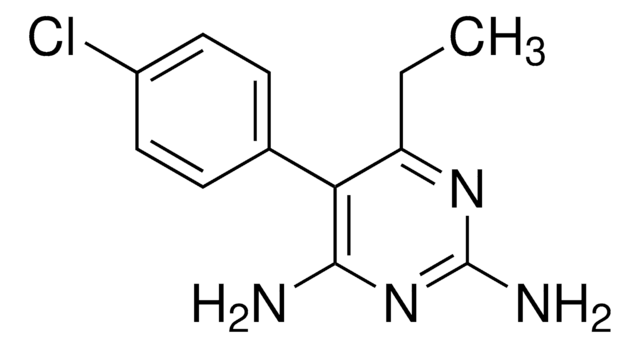
PHR1896
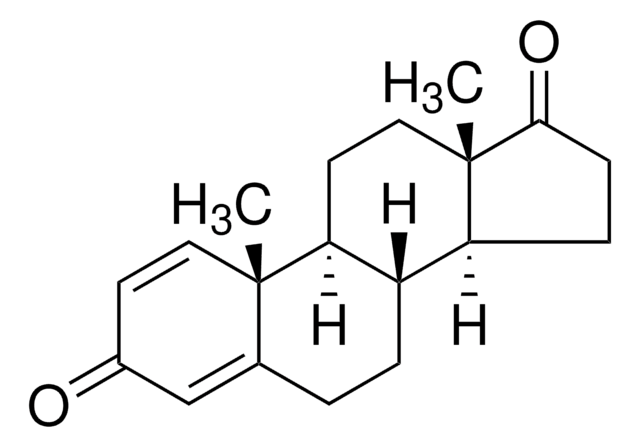
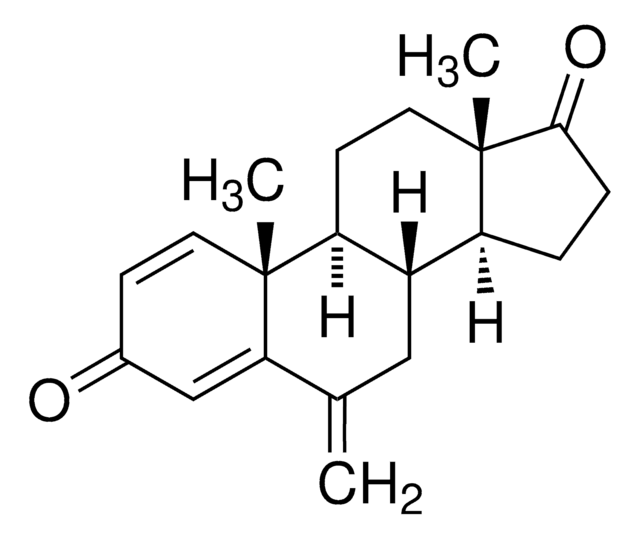
Exemestane Related Compound D
Pharmaceutical Secondary Standard; Certified Reference Material
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Código UNSPSC:
41116107
NACRES:
NA.24
Productos recomendados
grado
certified reference material
pharmaceutical secondary standard
Nivel de calidad
familia API
exemestane
CofA
current certificate can be downloaded
envase
pkg of 30 mg
aplicaciones
pharmaceutical
formato
neat
temp. de almacenamiento
2-8°C
Descripción general
Exemestane Related Compound D is an impurity of the steroidal anticancer drug exemestane. Exemestane belongs to the class of antiestrogens known as aromatase inhibitors and it is commonly used for the treatment of breast cancer.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Aplicación
Exemestane may be used as a pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by chromatography.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Nota de análisis
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
Otras notas
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Nota al pie de página
To see an example of a Certificate of Analysis for this material enter LRAB0340 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
Producto relacionado
Referencia del producto
Descripción
Precios
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Analytical method validation for HPLC assay of oral anticancer drug exemestane
Yavuz B
Journal of Pharmaceutical Sciences, 32(1), 15-15 (2007)
Exemestane: a review of its clinical efficacy and safety
L?nning PE
Breast (Edinburgh, Scotland), 10(3), 198-208 (2001)
A novel validated stability-indicating RP-HPLC method for the determination of Exemestane (steroidal aromatase inhibitor)
Mukthinuthalapati MA and Bukkapatnam V
Journal of Bioequivalence & Bioavailability, 7(6), 288-288 (2015)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico