PHR1752
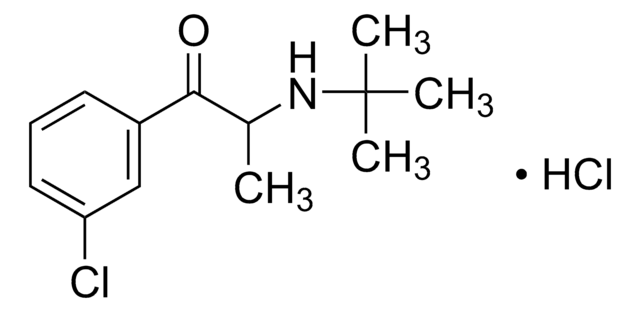
Bupropion Hydrochloride Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
Sinónimos:
2-(tert-Butylamino)-4′-chloropropiophenone hydrochloride, 2-(tert-butylamino)-3’-bromopiophenone hydrochloride
About This Item
Productos recomendados
grado
certified reference material
pharmaceutical secondary standard
Nivel de calidad
Agency
traceable to USP 1078744
familia API
bupropion
CofA
current certificate can be downloaded
envase
pkg of 30 mg
técnicas
HPLC: suitable
gas chromatography (GC): suitable
aplicaciones
pharmaceutical (small molecule)
Formato
neat
temp. de almacenamiento
2-8°C
cadena SMILES
Clc1ccc(cc1)C(=O)C(NC(C)(C)C)C.Cl
InChI
1S/C13H18ClNO.ClH/c1-9(15-13(2,3)4)12(16)10-5-7-11(14)8-6-10;/h5-9,15H,1-4H3;1H
Clave InChI
DGJAXLCGRQSHOO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Bupropion hydrochloride (BUP) and its derivatives belong to a chemical class of aminoketones and are known for their antidepressant abilities. BUP selectively inhibits the neuronal reabsorption of catecholamines (noradrenalin and dopamine), has minimal effect on the recapture of indolamines (serotonin) and no inhibitory effect on monoamine oxidase. It is used as the non-nicotine pharmacological therapy for combating smoking in controlled release form.
Bupropion hydrochloride (BUP) may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by spectrophotometric and chromatographic techniques.
Aplicación
Nota de análisis
Otras notas
Nota al pie de página
Productos recomendados
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico


![4-[(1RS)-2-(tert-Butylamino)-1-hydroxyethyl]-2-ethylphenol hydrochloride certified reference material, pharmaceutical secondary standard](/deepweb/assets/sigmaaldrich/product/images/399/698/c88a0004-aea6-4f13-9d6a-09d648ff9eb0/640/c88a0004-aea6-4f13-9d6a-09d648ff9eb0.jpg)