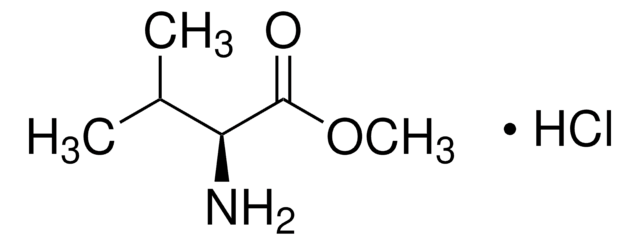
06054
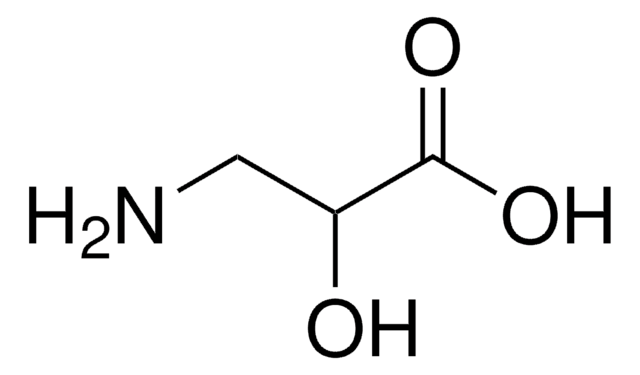
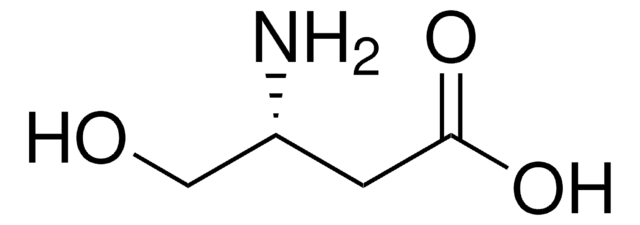
L-Isoserine
≥98.0% (TLC)
Sinónimos:
(S)-2-Hydroxy-β-alanine, (S)-3-Amino-2-hydroxypropionic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C3H7NO3
Número de CAS:
Peso molecular:
105.09
Número MDL:
Código UNSPSC:
12352209
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Análisis
≥98.0% (TLC)
formulario
powder
idoneidad de la reacción
reaction type: solution phase peptide synthesis
color
white to off-white
mp
197-198 °C
aplicaciones
peptide synthesis
cadena SMILES
NC[C@H](O)C(O)=O
InChI
1S/C3H7NO3/c4-1-2(5)3(6)7/h2,5H,1,4H2,(H,6,7)/t2-/m0/s1
Clave InChI
BMYNFMYTOJXKLE-REOHCLBHSA-N
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Mei-Xiang Wang et al.
The Journal of organic chemistry, 70(7), 2439-2444 (2005-03-25)
[reaction: see text] Biotransformations of a number of differently substituted and configured oxiranecarbonitriles using Rhodococcus sp. AJ270, a microbial whole-cell catalyst that contains nitrile hydratase/amidase, were studied. While almost all trans-configured 3-aryl-2-methyloxiranecarbonitriles and 2,3-dimethyl-3-phenyloxiranecarbonitrile were efficiently hydrated by the action
Microbial resolution of 2-hydroxy-3-nitropropionic acid for synthesis of optically active isoserine.
Y Yasohara et al.
Bioscience, biotechnology, and biochemistry, 65(5), 1258-1260 (2001-07-07)
The biocatalytic stereoselective hydrolysis of 2-hydroxy-3-nitropropionic acid esters was studied. Forty enzymes and three hundred microorganism strains were examined for their ability to hydrolyze ethyl 2-hydroxy-3-nitropropionic acid. Nocardia globerula IFO13150 gave n-butyl (R)-2-hydroxy-3-nitropropionate with a 92% enantiomeric excess (ee) and
Jan Cz Dobrowolski et al.
Physical chemistry chemical physics : PCCP, 12(36), 10818-10830 (2010-07-10)
The IR low-temperature Ar and Kr matrix spectra of l-isoserine were registered for the first time and interpreted by means of the anharmonic DFT frequencies calculated at the B3LYP/aug-cc-pVTZ and B3LYP/aug-cc-pVDZ levels. 54 l-isoserine conformers were predicted to be stable
Alexander Titz et al.
Bioorganic & medicinal chemistry, 18(1), 19-27 (2009-12-02)
The selectin-leukocyte interaction is the initial event in the early inflammatory cascade. This interplay proceeds via the terminal tetrasaccharide sialyl Lewis(x) (sLe(x)), present on physiological selectin ligands and E- and P-selectins located on the endothelial surface. Blocking this process is
J Du et al.
Nucleosides & nucleotides, 17(1-3), 1-13 (1998-08-26)
Asymmetric synthesis of N-substituted oxazolidinyl nucleosides has been accomplished from L-isoserine, trans- and cis-Oxazolidine intermediates (4 and 5) were stereoselectively constructed from N-protected L-isoserine with a menthoxycarbonyl group by the condensation with benzoyloxy acetaldehyde dimethyl acetal in a ratio of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico