MABN71
Anti-GluR2 Antibody, clone L21/32
clone L21/32, from mouse
Sinónimos:
glutamate receptor, ionotropic, AMPA 2, glutamate receptor 2, Glutamate receptor ionotropic, AMPA 2, AMPA-selective glutamate receptor 2
About This Item
WB
western blot: suitable
Productos recomendados
origen biológico
mouse
Nivel de calidad
forma del anticuerpo
purified antibody
tipo de anticuerpo
primary antibodies
clon
L21/32, monoclonal
reactividad de especies
rat
reactividad de especies (predicha por homología)
mouse (based on 100% sequence homology), human (immunogen homology)
técnicas
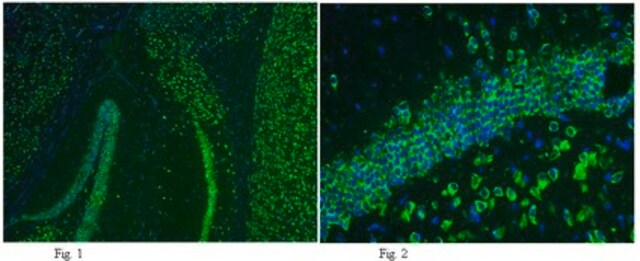
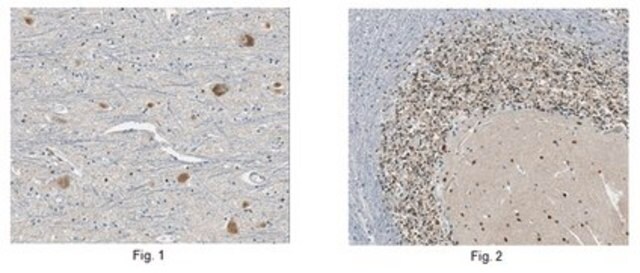
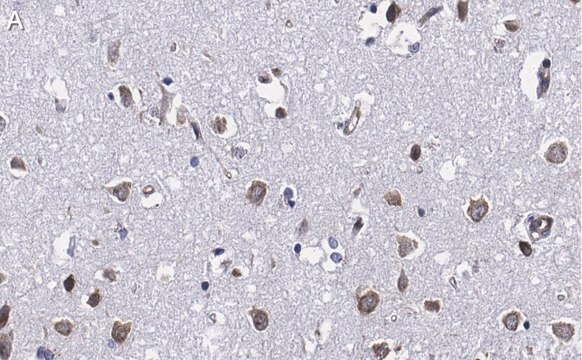
immunohistochemistry: suitable
western blot: suitable
isotipo
IgG1κ
Nº de acceso NCBI
Nº de acceso UniProt
Condiciones de envío
wet ice
modificación del objetivo postraduccional
unmodified
Información sobre el gen
human ... GRIA2(2891)
Descripción general
Inmunógeno
Aplicación
Neuroscience
Neuroscience
Neurodegenerative Diseases
Neurotransmitters & Receptors
Calidad
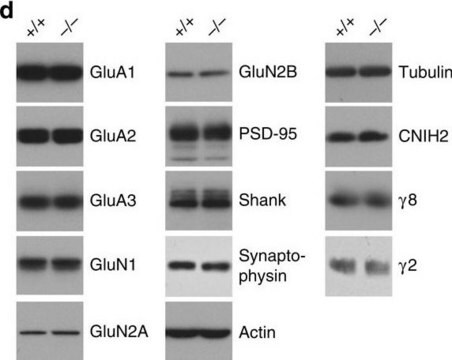
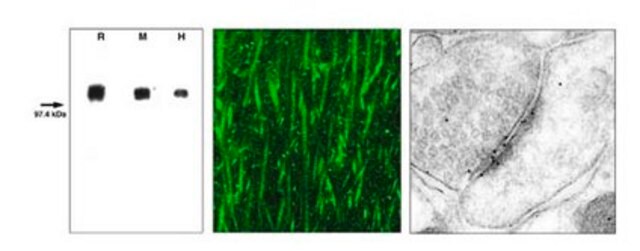
Western Blot Analysis: 0.5 µg/mL of this antibody detected GluR2 on 10 µg of rat brain membrane tissue lysate.
Descripción de destino
Forma física
Almacenamiento y estabilidad
Nota de análisis
Rat brain membrane tissue lysate
Otras notas
Cláusula de descargo de responsabilidad
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Opcional
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico