R-006
Risperidone solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grado
certified reference material
Nivel de calidad
Formulario
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
envase
ampule of 1 mL
fabricante / nombre comercial
Cerilliant®
concentración
1.0 mg/mL in methanol
técnicas
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicaciones
clinical testing
Formato
single component solution
temp. de almacenamiento
−20°C
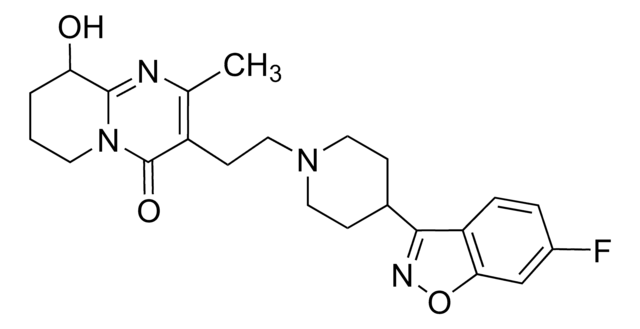
cadena SMILES
CC1=C(CCN2CCC(CC2)c3noc4cc(F)ccc34)C(=O)N5CCCCC5=N1
InChI
1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3
Clave InChI
RAPZEAPATHNIPO-UHFFFAOYSA-N
Información sobre el gen
human ... DRD2(1813) , HTR2A(3356) , HTR2C(3358)
Categorías relacionadas
Descripción general
Información legal
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Órganos de actuación
Eyes,Central nervous system
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
49.5 °F - closed cup
Punto de inflamabilidad (°C)
9.7 °C - closed cup
Listados normativos
Los listados normativos se proporcionan para los productos químicos principalmente. Para los productos no químicos sólo se puede proporcionar información limitada. Si no hay ninguna entrada, significa que ninguno de los componentes está en la lista. Es obligación del usuario garantizar el uso seguro y legal del producto.
EU REACH Annex XVII (Restriction List)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico