P-011
(+)-Propoxyphene solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grado
certified reference material
Nivel de calidad
Formulario
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
envase
ampule of 1 mL
fabricante / nombre comercial
Cerilliant®
drug control
Narcotic Licence Schedule A (Switzerland); estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
concentración
1.0 mg/mL in acetonitrile
técnicas
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicaciones
forensics and toxicology
Formato
single component solution
temp. de almacenamiento
2-8°C
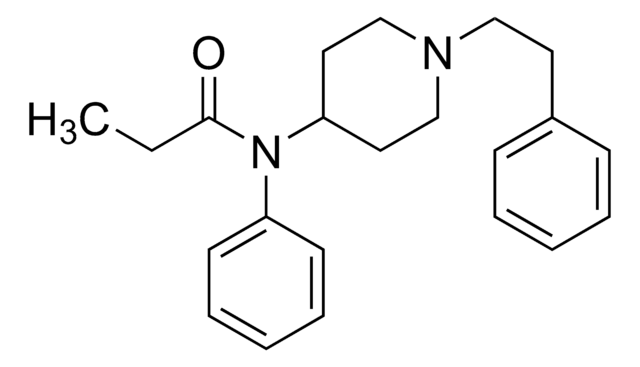
cadena SMILES
CCC(=O)O[C@@](Cc1ccccc1)([C@H](C)CN(C)C)c2ccccc2
InChI
1S/C22H29NO2/c1-5-21(24)25-22(18(2)17-23(3)4,20-14-10-7-11-15-20)16-19-12-8-6-9-13-19/h6-15,18H,5,16-17H2,1-4H3/t18-,22+/m1/s1
Clave InChI
XLMALTXPSGQGBX-GCJKJVERSA-N
Información sobre el gen
human ... OPRM1(4988)
Descripción general
Información legal
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
35.6 °F - closed cup
Punto de inflamabilidad (°C)
2 °C - closed cup
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| P-011-1ML | 4061834249214 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico