A-166
Adalimumab solution
10.0 mg/mL (12.5 mM Histidine Buffer), certified reference material, Cerilliant®
About This Item
Productos recomendados
grado
certified reference material
Nivel de calidad
formulario
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
envase
ampule of 0.25 mL
fabricante / nombre comercial
Cerilliant®
concentración
10.0 mg/mL (12.5 mM Histidine Buffer)
técnicas
liquid chromatography (LC): suitable
aplicaciones
clinical testing
formato
single component solution
temp. de almacenamiento
−20°C
Descripción general
Aplicación
Características y beneficios
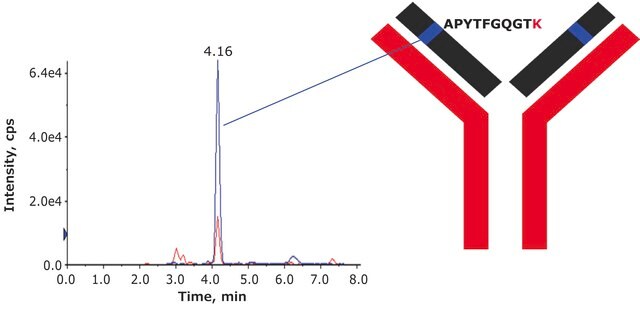
- Adalimumab (Humira) content is quantitatively measured by amino acid analysis (AAA).
- Every raw material employed has been identified and comprehensively characterized using a variety of analytical methods.
- The fill volume is gravimetrically verified at various stages during the dispensing process using qualified and calibrated balances.
- Long-term stability has been evaluated under freezer storage conditions (-10 °C to -25 °C).
Nota de preparación
- Thaw the product in a refrigerator or at room temperature and ensure thorough mixing before use.
- Avoid refreezing the product once thawed.
- When spiking into a matrix or diluting to required concentrations, it is recommended to quantitatively transfer the appropriate volume using established laboratory practices.
Otras notas
Información legal
Producto relacionado
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
A complete SEC-UV workflow for the characterization of mAb monomers, aggregates, and fragments using Zenix® and Zenix®-C SEC columns, including system suitability testing and forced pH and temperature stress studies.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico