D4407
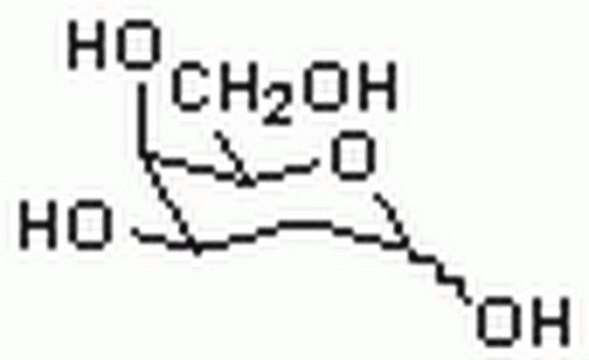
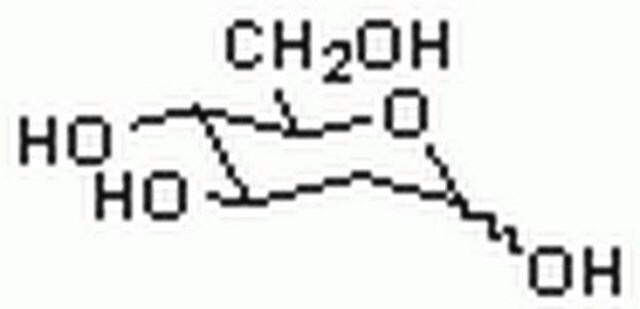
2-Deoxy-D-galactose
98%
Sinónimos:
2-Deoxy-D-lyxohexose
About This Item
Productos recomendados
Análisis
98%
formulario
powder
actividad óptica
[α]20/D +59.7°, c = 2 in H2O
color
white to off-white
mp
107 - 110 °C ((225 - 230 °F))
107-110 °C (lit.)
cadena SMILES
OC[C@H]1OC(O)C[C@@H](O)[C@H]1O
InChI
1S/C6H12O5/c7-2-4-6(10)3(8)1-5(9)11-4/h3-10H,1-2H2/t3-,4-,5?,6-/m1/s1
Clave InChI
PMMURAAUARKVCB-DUVQVXGLSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- FUT1-mediated terminal fucosylation acts as a new target to attenuate renal fibrosis.: This research investigates the role of 2-deoxy-D-galactose in modulating terminal fucosylation processes, revealing potential therapeutic pathways for treating renal fibrosis and enhancing the understanding of kidney disease mechanisms (Luo et al., 2023).
- 2-D-gal Targets Terminal Fucosylation to Inhibit T-cell Response in a Mouse Skin Transplant Model.: Highlights the immunomodulatory potential of 2-deoxy-D-galactose in transplant medicine, showing how it can inhibit T-cell responses and contribute to the success of skin grafts, pointing towards new immunosuppressive treatments (Mao et al., 2023).
- Inhibition of Aberrant α(1,2)-Fucosylation at Ocular Surface Ameliorates Dry Eye Disease.: Explores the therapeutic effects of 2-deoxy-D-galactose in treating dry eye disease by modulating specific fucosylation pathways, potentially opening new avenues for ocular surface treatment strategies (Yoon et al., 2021).
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico