777676
Graphene oxide dispersion
4 mg/mL,dispersion in H2O, avg. no. of layers, 1
Sinónimos:
GO dispersion in H2O
About This Item
Productos recomendados
product name
Graphene oxide, 4 mg/mL, dispersion in H2O, avg. no. of layers, 1
descripción
dispersibility: Polar solvents
Nivel de calidad
formulario
dispersion in H2O
Características
avg. no. of layers 1 measured in 0.5mg/mL (>95%)
avg. no. of layers 1
características de los productos alternativos más sostenibles
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentración
4 mg/mL
categoría alternativa más sostenible
, Enabling
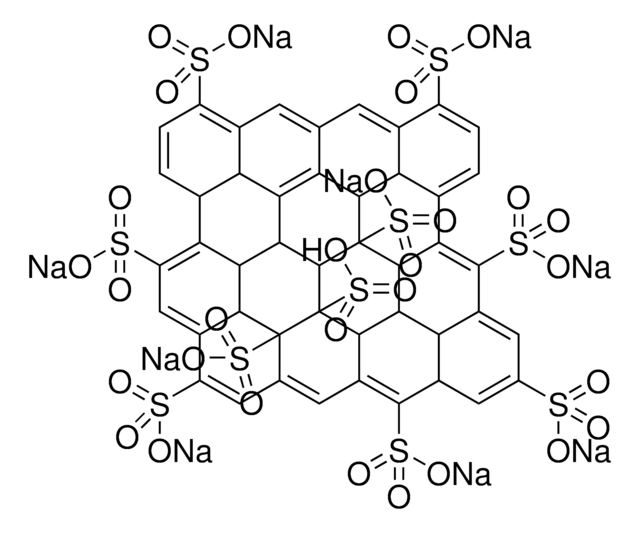
cadena SMILES
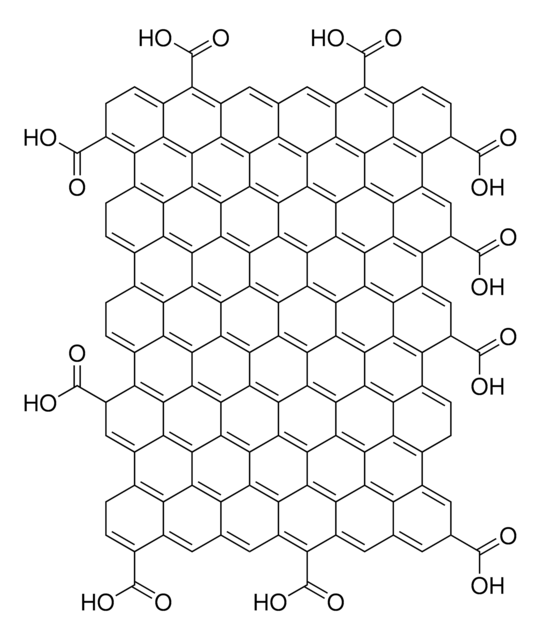
O=C(O)C1C2=C3C4=C5C6=C7C8=C9C%10=C%11C(C%12=C%13C%10=C%14C8=C%15C6=C%16C4=C%17C2=CC(C(O)=O)C%18=C%17C%19=C%16C%20=C%15C%21=C%14C%22=C%13C(C%23=C%24C%22=C%25C%21=C%26C%20=C%27C%19=C%28C%18=CC(C(O)=O)C%29=C%28C%30=C%27C%31=C%26C%32=C%25C%33=C%24C(C%34=C%35C
InChI
1S/C140H42O20/c141-131(142)26-13-23-15-44-62(140(159)160)45-16-24-14-40-31(132(143)144)5-1-29-41-20-48(135(149)150)56-33-7-3-28-27-2-6-32-55-37(133(145)146)11-9-35-60(138(155)156)42-17-25-18-43-61(139(157)158)36-10-12-38(134(147)148)58-46-21-50(137(153)154)59-47-22-49(136(151)152)57-34-8-4-30-39(19-26)51(23)78-72(44)88-75(45)80-52(24)79(54(29)40)95-71(41)83(56)101-93-69(33)64(28)91-90-63(27)68(32)92-86(66(35)55)73(42)81-53(25)82-74(43)87(67(36)58)96-76(46)85(59)103-97-77(47)84(57)102-94-70(34)65(30)89(78)105-104(88)115-98(80)111(95)116(101)126-122-110(93)107(91)120-119-106(90)108(92)99(81)114-100(82)112(96)118(103)128(124(114)119)123-113(97)117(102)127(130(122)129(120)123)121(109(94)105)125(115)126/h2,5,7-10,12-22,26,38,48-50H,1,3-4,6,11H2,(H,141,142)(H,143,144)(H,145,146)(H,147,148)(H,149,150)(H,151,152)(H,153,154)(H,155,156)(H,157,158)(H,159,160)
Clave InChI
VTWITIAIMADGRM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
Almacenamiento y estabilidad
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Graphene oxide is a unique material that can be viewed as a single monomolecular layer of graphite with various oxygen containing functionalities such as epoxide, carbonyl, carboxyl and hydroxyl groups.
Carbon nanomaterials (CNMs), such as single-walled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs), and graphene (Figure 1), have diverse commercial applications including lighter and stronger composite materials, improved energy storage devices, more sensitive sensors, and smaller transistors.
Professor Ebrahimi and Professor Robinson (Pennsylvania State University, USA) summarize recent advances in the synthesis of these 2D materials, resulting material properties, and related applications in biosensing of neurotransmitters, metabolites, proteins, nucleic acids, bacterial cells, and heavy metals.
Recent demand for electric and hybrid vehicles, coupled with a reduction in prices, has caused lithium-ion batteries (LIBs) to become an increasingly popular form of rechargeable battery technology.
Contenido relacionado
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico