420352
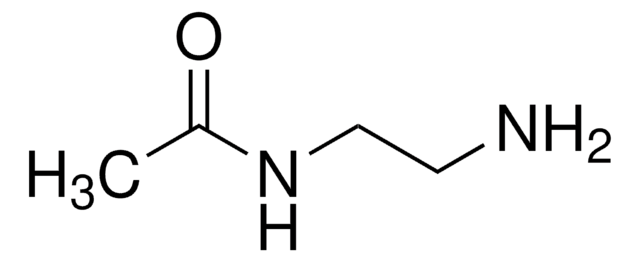
Ethylenediamine diacetate
98%
Sinónimos:
Ethanediammonium diacetate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
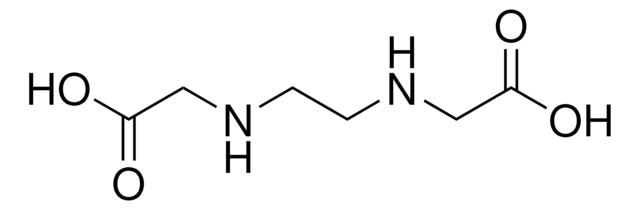
NH2CH2CH2NH2 · 2CH3COOH
Número de CAS:
Peso molecular:
180.20
Beilstein:
5444394
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
98%
mp
118-120 °C (lit.)
grupo funcional
amine
carboxylic acid
cadena SMILES
CC(O)=O.CC(O)=O.NCCN
InChI
1S/C2H8N2.2C2H4O2/c3-1-2-4;2*1-2(3)4/h1-4H2;2*1H3,(H,3,4)
Clave InChI
SAXQBSJDTDGBHS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
- Catalytic Synthesis: Utilization of Ethylenediamine Diacetate as an effective catalyst for synthesizing specific thioxothiazolidin-4-one, important for organic chemists and pharmacological studies aiming at new drug development (S Holota, A Lozynskyi, Y Konechnyi, Y Shepeta, 2021).
- Reaction of New Amides: Exploration of reactions between new N-(2,2-Dichloro-1-cyanoethenyl)amides and aliphatic amines facilitated by Ethylenediamine Diacetate, relevant for medicinal chemistry and synthesis (OV Shablykin, SA Chumachenko, 2021).
- Microwave-Assisted Organic Synthesis: Application of Ethylenediamine Diacetate in microwave-assisted synthesis processes, which is valuable for accelerating chemical reactions in drug synthesis and material processing (P Martín-Acosta, G Feresin, A Tapia, 2016).
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Ethylenediamine diacetate (EDDA)-catalyzed one-pot synthesis of tetrahydroquinolines by domino Knoevenagel/hetero Diels-Alder reactions from 1, 3-dicarbonyls.
Lee YR and Hung TV.
Tetrahedron, 67(49), 9627-9634 (2011)
Efficient and general method for the synthesis of benzopyrans by ethylenediamine diacetate-catalyzed reactions of resorcinols with α, β-unsaturated aldehydes. One step synthesis of biologically active (?)-confluentin and (?)-daurichromenic acid.
Lee YR, et al.
Tetrahedron Letters, 46(44), 7539-7543 (2005)
Kusum Vats et al.
Journal of labelled compounds & radiopharmaceuticals, 60(9), 431-438 (2017-05-26)
Targeted delivery of chemotherapeutic drug at the tumor site enhances the efficacy with minimum systemic exposure. Towards this, drugs conjugated with peptides having affinity towards a particular molecular target are recognized as affective agents for targeted chemotherapy. Thus, in the
Euan Pyle et al.
Cell chemical biology, 25(7), 840-848 (2018-04-24)
The role of membrane lipids in modulating eukaryotic transporter assembly and function remains unclear. We investigated the effect of membrane lipids in the structure and transport activity of the purine transporter UapA from Aspergillus nidulans. We found that UapA exists
Sergii Yakunin et al.
Nature communications, 12(1), 981-981 (2021-02-14)
Traditional fluorescence-based tags, used for anticounterfeiting, rely on primitive pattern matching and visual identification; additional covert security features such as fluorescent lifetime or pattern masking are advantageous if fraud is to be deterred. Herein, we present an electrohydrodynamically printed unicolour
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| 420352-1G | |
| 420352-5G | 4061832093000 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico