392529
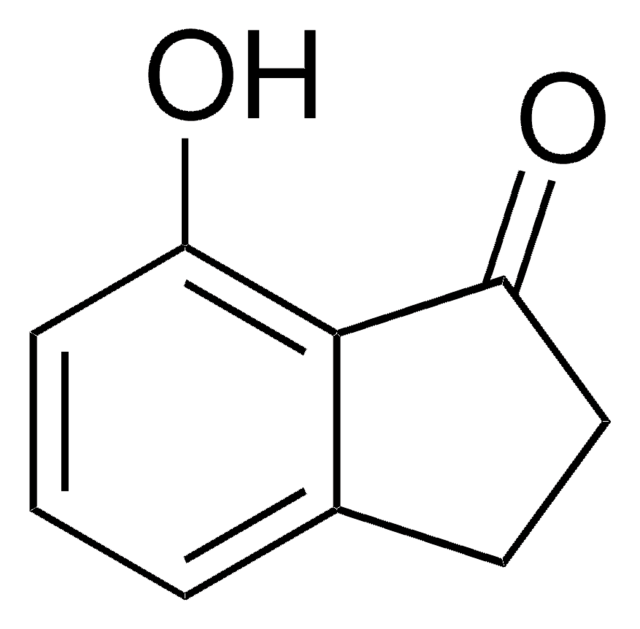
4-Methoxy-1-indanone
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H10O2
Número de CAS:
Peso molecular:
162.19
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Análisis
99%
bp
115-120 °C/0.5 mmHg (lit.)
mp
105-107 °C (dec.) (lit.)
grupo funcional
ketone
cadena SMILES
COc1cccc2C(=O)CCc12
InChI
1S/C10H10O2/c1-12-10-4-2-3-7-8(10)5-6-9(7)11/h2-4H,5-6H2,1H3
Clave InChI
BTYSYELHQDGJAB-UHFFFAOYSA-N
Descripción general
4-Methoxy-1-indanone, a benzo-fused ketone is a 1-indanone derivative. Its synthesis has been reported.
Aplicación
4-Methoxy-1-indanone is suitable for use in the comparative study of the effect of different substituents on various benzo-fused ketones on the reaction kinetics by studying the biocatalysed oxidation reaction.
It may be used in the following studies:
It may be used in the following studies:
- Synthesis of isomeric mixture of oximes.
- As a starting material in the synthesis of benzo-fused indolizidine and 4-methoxy-1-indanyl compound.
- Synthesis of 4-methoxy-5-nitro-1-indanone by nitration reaction.
- As one of the reactant in the synthesis of E-2-chloro-8-methyl-3-[(4′-methoxy-1′-indanoyl)-2′-methyliden]-quinoline (IQ), a quinoline derivative.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
W H Pearson et al.
The Journal of organic chemistry, 65(21), 7158-7174 (2000-10-14)
The intramolecular capture of benzocyclobutyl, benzocyclopentyl, and benzocyclohexyl carbocations 7 by azides produces spirocyclic aminodiazonium ions 8, which undergo 1,2-C-to-N rearrangement with loss of dinitrogen to produce benzo-fused iminium ions resulting from either aryl (9) or alkyl (10) migration to
Beckmann rearrangements of 1-indanone oxime derivatives using aluminum chloride and mechanistic considerations.
Lee BS, et al.
Bull. Korean Chem. Soc., 21(9), 860-866 (2000)
Juan Rodrigues et al.
Memorias do Instituto Oswaldo Cruz, 104(6), 865-870 (2009-10-31)
E-2-chloro-8-methyl-3-[(4'-methoxy-1'-indanoyl)-2'-methyliden]-quinoline (IQ) is a new quinoline derivative which has been reported as a haemoglobin degradation and ss-haematin formation inhibitor. The haemoglobin proteolysis induced by Plasmodium parasites represents a source of amino acids and haeme, leading to oxidative stress in infected
Enzymatic Baeyer-Villiger Oxidation of Benzo-Fused Ketones: Formation of Regiocomplementary Lactones.
Rioz-Martinez A, et al.
European Journal of Organic Chemistry, 2009(15), 2526-2532 (2009)
Ju-Ok Lim et al.
European journal of medicinal chemistry, 44(1), 322-331 (2008-04-15)
A series of bicyclic analogues having indan and tetrahydronaphthalene templates in the A-region were designed as conformationally constrained analogues of our previously reported potent TRPV1 antagonists (1, 3). The activities for rat TRPV1 of the conformationally restricted analogues were moderately
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico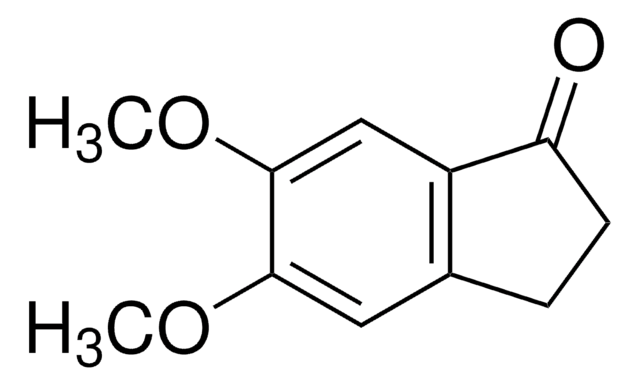

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)