326755
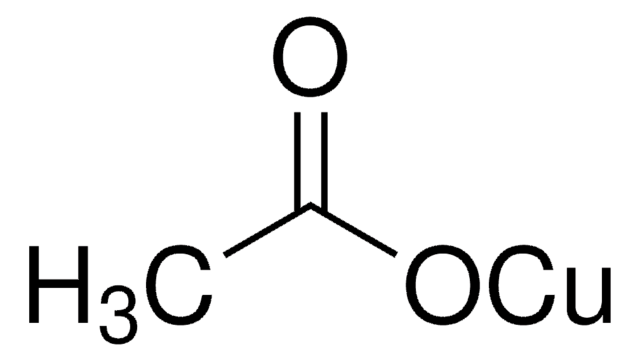
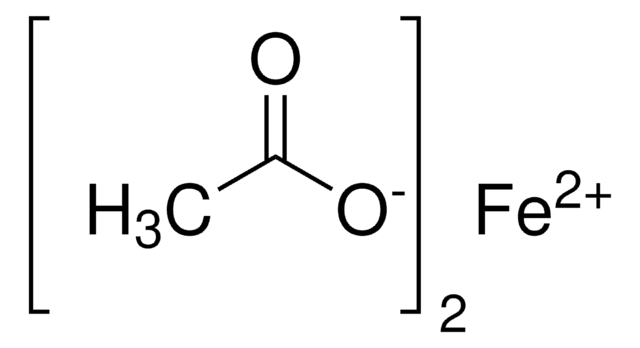
Copper(II) acetate
98%
Sinónimos:
Cupric acetate
About This Item
Productos recomendados
grado
for analytical purposes
densidad de vapor
6.9 (vs air)
Ensayo
98%
Formulario
powder or crystals
idoneidad de la reacción
reaction type: click chemistry
características de los productos alternativos más sostenibles
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
categoría alternativa más sostenible
, Aligned
cadena SMILES
CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
Clave InChI
OPQARKPSCNTWTJ-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Copper(II) acetate also known as cupric acetate, can be used as a catalyst in various processes in the field of greener chemistry. It is particularly useful in cross-coupling reactions, where it can promote the formation of carbon-carbon or carbon-heteroatom bonds, without the need for hazardous reagents or solvents
Aplicación
Copper-catalyzed reductive amination of aromatic and aliphatic ketones with anilines using environmental-friendly molecular hydrogen
Copper(II) acetate is used as a catalyst:
- In the N-arylation of α-amino esters with p-tolylboronic acid to synthesize biaryls via cross-coupling reactions
- In the the synthesis of substituted isoxazole derivatives
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Código de clase de almacenamiento
8B - Non-combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
does not flash
Punto de inflamabilidad (°C)
does not flash
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico