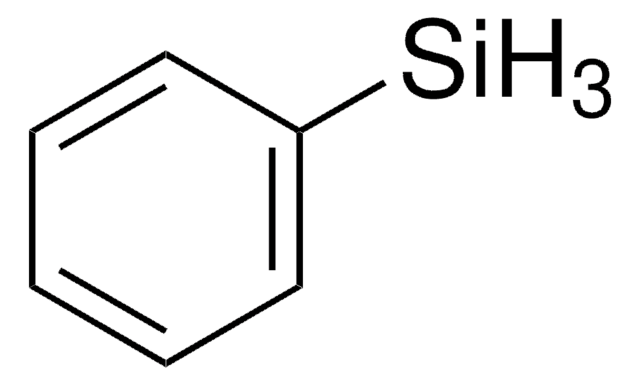
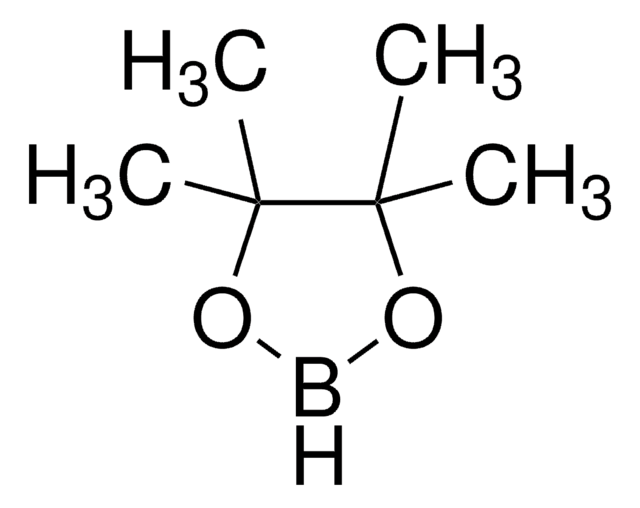
324647
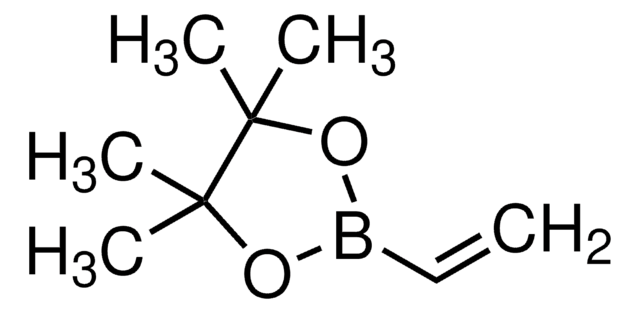
Allylboronic acid pinacol ester
97%
Sinónimos:
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4,4,5,5-tetramethyl-2-(2-propen-1-yl)-1,3,2-dioxaborolane, 4,4,5,5-tetramethyl-2-(2-propenyl)-1,3,2-dioxaborolane, Allyl pinacol boronate, Pinacol allylboronate, Pinacolyl 2-propenylboronate
About This Item
Productos recomendados
Nivel de calidad
Ensayo
97%
índice de refracción
n20/D 1.4268 (lit.)
bp
50-53 °C/5 mmHg (lit.)
densidad
0.896 g/mL at 25 °C (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
CC1(C)OB(CC=C)OC1(C)C
InChI
1S/C9H17BO2/c1-6-7-10-11-8(2,3)9(4,5)12-10/h6H,1,7H2,2-5H3
Clave InChI
YMHIEPNFCBNQQU-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
Aplicación
- Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions and olefin metathesis
- Intermolecular radical additions
- Allylboration of aldehydes catalyzed by chiral spirobiindane diol (SPINOL) based phosphoric acids
- Cobalt-catalyzed regioselective hydrovinylation of dienes with alkenes
- Nucleic acid-templated energy transfer leading to a photorelease reaction
- Stereoselective indium-catalyzed Hosomi-Sakurai reactions
Reagent used in Preparation of
- Cyclic sulfone hydroxyethylamines as BACE1 inhibitors for reduction of Amyloid β-Peptides
- Transmetalation of carbene Ru iodide with Ag carboxylates to give C-H-activated Ru carbene complexes as catalysts for Z-selective olefin metathesis
- Allylboronation reagent for the preparation of allylic alcohols, and homoallylic amines.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Flam. Liq. 3
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
114.8 °F - closed cup
Punto de inflamabilidad (°C)
46 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico