311073
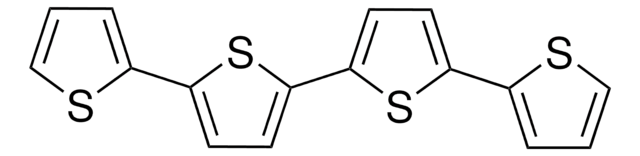
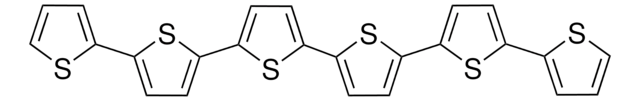
2,2′:5′,2′′-Terthiophene
99%
Sinónimos:
α-Terthienyl, 2,5-Di(2-thienyl)thiophene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C12H8S3
Número de CAS:
Peso molecular:
248.39
Beilstein:
178604
Número MDL:
Código UNSPSC:
12352103
ID de la sustancia en PubChem:
NACRES:
NA.23
Productos recomendados
Ensayo
99%
mp
93-95 °C (lit.)
cadena SMILES
c1csc(c1)-c2ccc(s2)-c3cccs3
InChI
1S/C12H8S3/c1-3-9(13-7-1)11-5-6-12(15-11)10-4-2-8-14-10/h1-8H
Clave InChI
KXSFECAJUBPPFE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
2,2′:5′,2′′-Terthiophene (TTh) may be prepared by nickel catalysed coupling reaction of grignard′s reagent derived from 2-bromothiophene and magnesium. It generates singlet oxygen. In nature, it is found in the floral extract of Tagetes minuta and Echinops grijisii. It is known to be toxic to mosquitoes. It also exihibits antifungal activity.
Aplicación
3T can be combined with 3,4-ethylenedioxythiophene (EDOT) in a tetrabutylammonium perchlorate solution for use as an electrochromic copolymer for a wide range of applications like photovoltaics and polymer light emitting diodes (LEDs). It can also be used to form metal-organic based thin films with metals like aluminum, silver, and calcium which can potentially be used for optoelectronics based applications.
Electrochemical copolymerization of carbazole and TTh in sodium perchlorate/acetonitrile was reported. Electrochromic copolymer based on TTh and 3, 4-ethylenedioxythiophene has been reported. TTh acts as a monomer precursor for polythiophene and as a dopant for polycarbonate. It may function as a photosensitizer.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Synthesis and characterization of an electrochromic copolymer based on 2, 2′ : 5′ , 2″ -terthiophene and 3, 4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2(2), 133-141 (2012)
Man Jae Park et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(7), 2056-2062 (2012-01-18)
Excess-electron transfer (EET) in DNA has attracted wide attention owing to its close relation to DNA repair and nanowires. To clarify the dynamics of EET in DNA, a photosensitizing electron donor that can donate an excess electron to a variety
Journal of Non-Crystalline Solids, 164, 1263-1263 (1993)
Hui-Bog Noh et al.
Biomaterials, 33(9), 2600-2607 (2012-01-03)
A highly sensitive in vivo biosensor for glutathione disulfide (GSSG) is developed using covalently immobilized-glutathione reductase (GR) and -β-nicotinamide adenine dinucleotide phosphate (NADPH) on gold nanoparticles deposited on poly[2,2':5',2″-terthiophene-3'-(p-benzoic acid)] (polyTTBA). The fabricated biosensor was characterized with SEM, TEM, XPS
Synthesis and characterization of an electrochromic copolymer based on 2,2':5',"-terthiophene and 3,4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2, 133-141 (2012)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico


![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)