286958
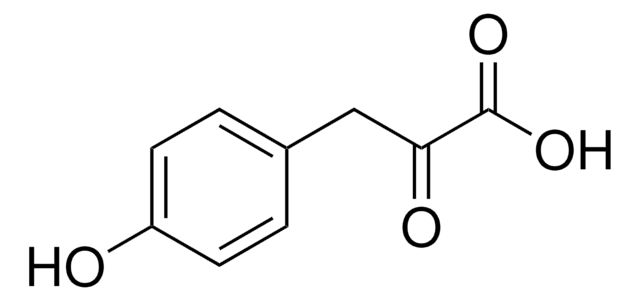
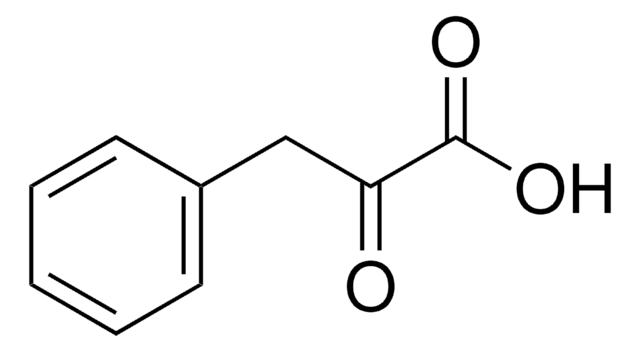
Phenylpyruvic acid
98%
Sinónimos:
2-Oxo-3-phenylpropanoic acid, 2-Oxo-3-phenylpropionic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH2COCOOH
Número de CAS:
Peso molecular:
164.16
Beilstein:
2207312
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
98%
mp
150-154 °C (lit.)
grupo funcional
carboxylic acid
ketone
phenyl
temp. de almacenamiento
−20°C
cadena SMILES
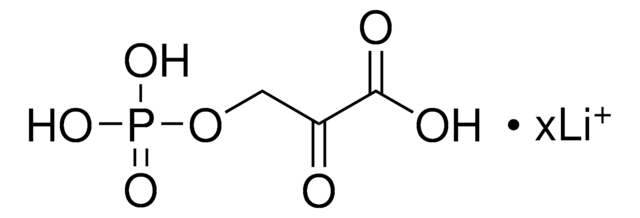
OC(=O)C(=O)Cc1ccccc1
InChI
1S/C9H8O3/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5H,6H2,(H,11,12)
Clave InChI
BTNMPGBKDVTSJY-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Phenylpyruvic acid reduces glucose-6-phosphate dehydrogenase activity without pre-incubation.
Aplicación
Phenylpyruvic acid was used in the synthesis of 3-phenyllactic acid (PLA) by lactate dehydrogenase.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Shuhuai Yu et al.
Biotechnology letters, 36(3), 627-631 (2013-11-20)
3-Phenyllactic acid (PLA) is an antimicrobial compound with broad and effective antimicrobial activity against both bacteria and fungi. Enzymatic production of PLA can be carried out from phenylpyruvic acid by lactate dehydrogenase (LDH); however, the enzymatic reaction is accompanied by
Taiki Fujii et al.
Biochimica et biophysica acta, 1814(12), 1669-1676 (2011-06-16)
We discovered the phenyllactate (PLA)-producing fungal strain Wickerhamia fluorescens TK1 and purified phenylpyruvate reductase (PPR) from fungal cell-free extracts. The PPR used both NADPH and NADH as cofactors with more preference for the former. The enzyme reaction as well as
Andrea Pereira Rosa et al.
Cellular and molecular neurobiology, 32(7), 1113-1118 (2012-04-06)
Phenylketonuria is a recessive autosomal disorder that is caused by a deficiency in the activity of phenylalanine-4-hydroxylase, which converts phenylalanine to tyrosine, leading to the accumulation of phenylalanine and its metabolites phenyllactic acid, phenylacetic acid, and phenylpyruvic acid in the
A Hargreaves et al.
Journal of photochemistry and photobiology. B, Biology, 89(2-3), 110-116 (2007-11-06)
Ultraviolet A (UVA) light (315-400 nm) is ubiquitously found in our environment and constitutes about 95% of the total solar UV; all UVC and most UVB being absorbed by the stratospheric ozone layer. Compared with UVB and C, UVA does
Faqing Tang et al.
Clinical biochemistry, 44(8-9), 711-718 (2011-03-16)
To search for markers of nasopharyngeal carcinoma (NPC) for diagnosis. Using gas chromatography and mass spectrometry, we evaluated 51 serum metabolites in 49 NPC, 37 throat cancer patients and 40 healthy controls. High metabolites were selected and confirmed in NPC
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico