270253
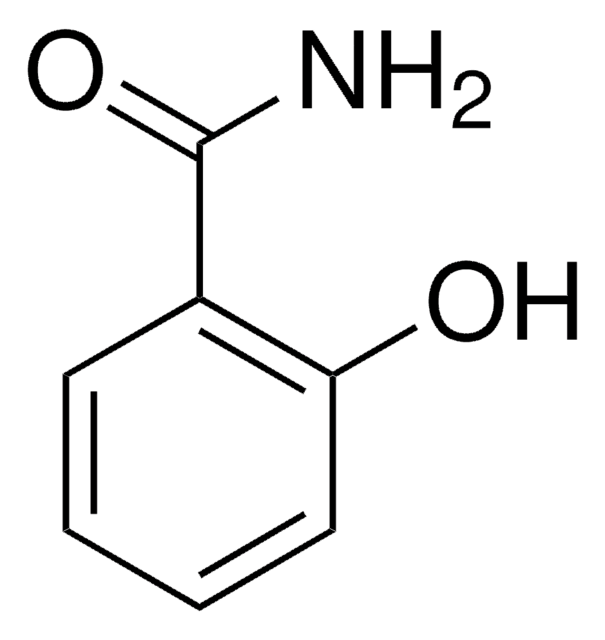
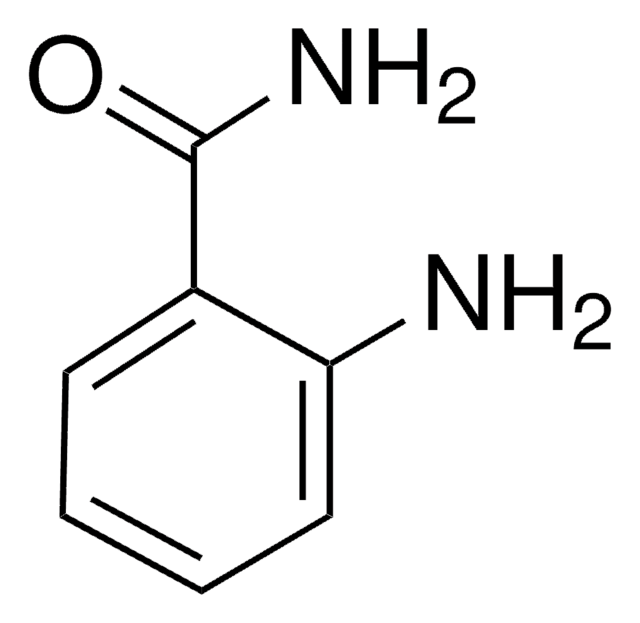
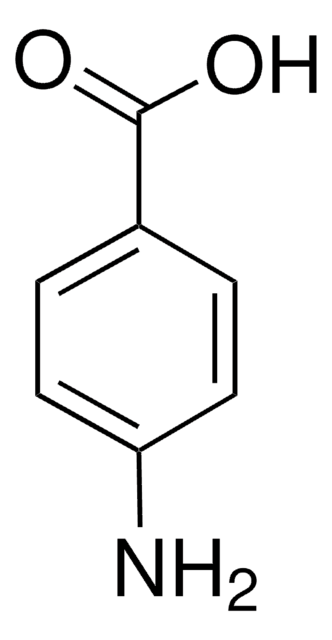
4-Hydroxybenzamide
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
HOC6H4CONH2
Número de CAS:
Peso molecular:
137.14
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
98%
mp
161-162 °C (lit.)
grupo funcional
amide
cadena SMILES
NC(=O)c1ccc(O)cc1
InChI
1S/C7H7NO2/c8-7(10)5-1-3-6(9)4-2-5/h1-4,9H,(H2,8,10)
Clave InChI
QXSAKPUBHTZHKW-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
The standard molar enthalpy of formation of 4-hydroxybenzamide was studied by micro- or macrocombustion calorimetry.
Aplicación
4-Hydroxybenzamide was used in the synthesis of balanol, a potent protein kinase C (PKC) inhibitor.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
R S Randad et al.
Bioorganic & medicinal chemistry, 4(9), 1471-1480 (1996-09-01)
A combination of structure-activity studies, kinetic analysis, X-ray crystallographic analysis, and modeling were employed in the design of a novel series of HIV-1 protease (HIV PR) inhibitors. The crystal structure of a complex of HIV PR with SRSS-2,5-bis[N-(tert-butyloxycarbonyl)amino]-3,4-dihydroxy-1, 6-diphenylhexane (1)
G D Hartman et al.
Bioorganic & medicinal chemistry letters, 9(6), 863-868 (1999-04-17)
A new series of potent, linearly-minimized, orally active, selective GPIIb/IIIa inhibitors is identified. Thus 15 (L-750,034) achieves interaction via a constrained, non-turned conformation that maintains the proper distance between its charged termini and full sulfonamide exosite interaction. The diminutive stature
C Emoto et al.
Xenobiotica; the fate of foreign compounds in biological systems, 37(12), 1408-1420 (2007-10-19)
CJ-036878, N-(3-phenethoxybenzyl)-4-hydroxybenzamide, was developed as an antagonist of the N-methyl-D-aspartate receptor NR2B subunit. Two dimeric metabolites, CJ-047710 and CJ-047713, were identified from the incubation mixture with CJ-036878 in human liver microsomes (HLM). The identification of the enzymes involved in the
Carlos E S Bernardes et al.
The journal of physical chemistry. A, 112(40), 10029-10039 (2008-09-13)
The energetics of the phenolic O-H bond in a series of 2- and 4-HOC 6H 4C(O)Y (Y = H, CH3, CH 2CH=CH2, C[triple bond]CH, CH2F, NH2, NHCH 3, NO2, OH, OCH3, OCN, CN, F, Cl, SH, and SCH3) compounds and
G D Smith et al.
Protein science : a publication of the Protein Society, 5(8), 1502-1511 (1996-08-01)
The structure of a symmetric T3R3f insulin hexamer, complexed with 4-hydroxybenzamide, has been determined using X-ray crystallographic techniques. Data were measured from six crystals grown in microgravity to a resolution of 1.4 A and the structure has been refined including
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| 270253-25G | 4061826141854 |
| 270253-5G | 4061826141861 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico