247820
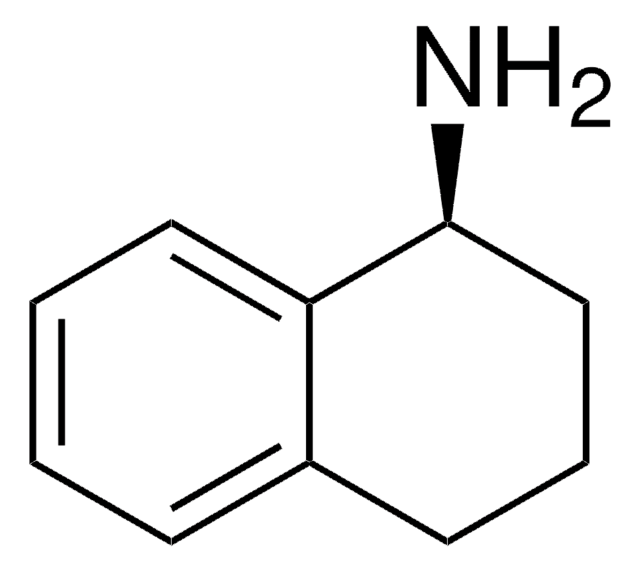
1,2,3,4-Tetrahydro-1-naphthylamine
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H13N
Número de CAS:
Peso molecular:
147.22
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
97%
Formulario
liquid
índice de refracción
n20/D 1.562 (lit.)
bp
246-247 °C/714 mmHg (lit.)
densidad
1.026 g/mL at 25 °C (lit.)
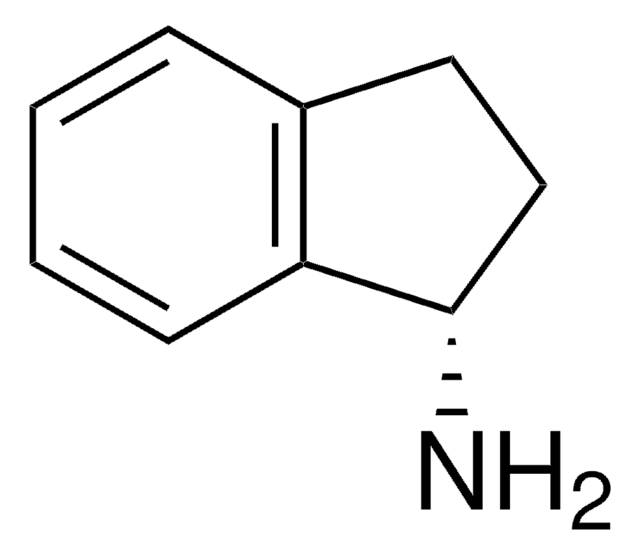
cadena SMILES
NC1CCCc2ccccc12
InChI
1S/C10H13N/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,4,6,10H,3,5,7,11H2
Clave InChI
JRZGPXSSNPTNMA-UHFFFAOYSA-N
Descripción general
(R)-1,2,3,4-Tetrahydro-1-naphthylamine is an efficient reagent for iodocyclization of 4-aryl-4-pentenoic acids.
Aplicación
1,2,3,4-Tetrahydro-1-naphthylamine has been used in the preparation of new chiral phosphine-aminophosphine ligands.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
235.4 °F - closed cup
Punto de inflamabilidad (°C)
113 °C - closed cup
Equipo de protección personal
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Modular Phosphine-Aminophosphine Ligands Based on Chiral 1, 2, 3, 4-Tetrahydro-1-naphthylamine Backbone: A New Class of Practical Ligands for Enantioselective Hydrogenations.
Qiu M, et al.
Advanced Synthesis & Catalysis, 350(17), 2683-2689 (2008)
Jürgen Haas et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 11(19), 5777-5785 (2005-07-23)
Lactonizations are important steps in many synthetic sequences. Substrate-controlled reactions that use chiral auxiliaries or chiral alkenes have already been studied in depth. This study focuses on stereoselective reagent-controlled iodolactonizations, by application of a new method that uses complexes of
J S Shin et al.
Biotechnology and bioengineering, 73(3), 179-187 (2001-03-21)
A kinetic resolution process for the production of chiral amines was developed using an enzyme-membrane reactor (EMR) and a hollow-fiber membrane contactor with (S)-specific omega-transaminases (omega-TA) from Vibrio fluvialis JS17 and Bacillus thuringiensis JS64. The substrate solution containing racemic amine
Noelia Madroñal et al.
Nature communications, 7, 10923-10923 (2016-03-19)
The hippocampus is critical for the acquisition and retrieval of episodic and contextual memories. Lesions of the dentate gyrus, a principal input of the hippocampus, block memory acquisition, but it remains unclear whether this region also plays a role in
Effects of isomers of apomorphines on dopamine receptors in striatal and limbic tissue of rat brain.
N S Kula et al.
Life sciences, 37(11), 1051-1057 (1985-09-16)
The optical isomers of apomorphine (APO) and N-propylnorapomorphine (NPA) were interacted with three biochemical indices of dopamine (DA) receptors in extrapyramidal and limbic preparations of rat brain tissue. There were consistent isomeric preferences for the R(-) configuration of both DA
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico