234893
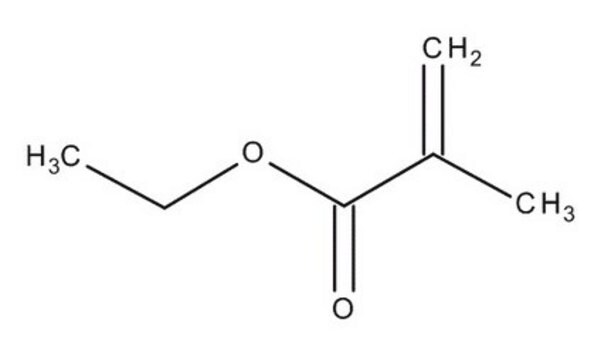
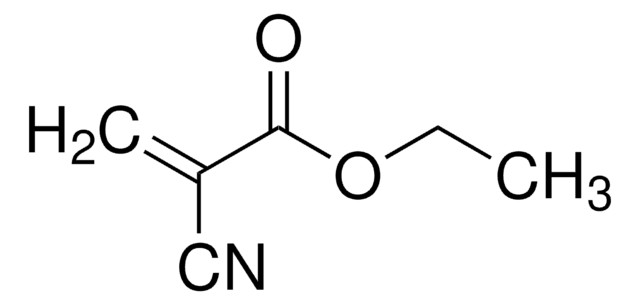
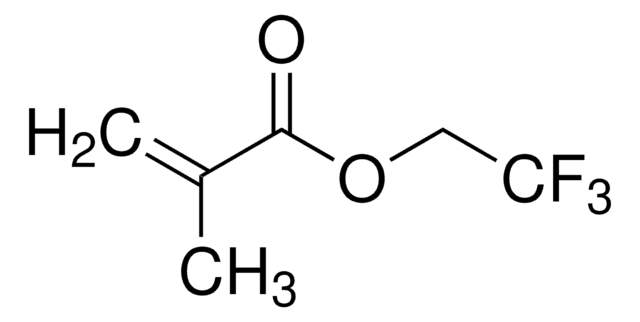
Ethyl methacrylate
contains 15-20 ppm monomethyl ether hydroquinone as inhibitor, 99%
Sinónimos:
2-Methyl-2-propenoic acid
About This Item
Productos recomendados
densidad de vapor
>3.9 (vs air)
Nivel de calidad
presión de vapor
15 mmHg ( 20 °C)
Análisis
99%
formulario
liquid
temp. de autoignición
771 °F
contiene
15-20 ppm monomethyl ether hydroquinone as inhibitor
índice de refracción
n20/D 1.413 (lit.)
bp
118-119 °C (lit.)
densidad
0.917 g/mL at 25 °C (lit.)
temp. de almacenamiento
2-8°C
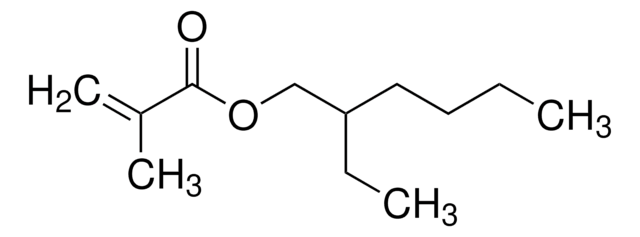
cadena SMILES
CCOC(=O)C(C)=C
InChI
1S/C6H10O2/c1-4-8-6(7)5(2)3/h2,4H2,1,3H3
Clave InChI
SUPCQIBBMFXVTL-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Ethyl methacrylate is a readily polymerizable monomer used for certain types of acrylic resins. The monomethyl ether hydroxyl quinone present in it is an inhibitor that prevents polymerization.
Aplicación
- To synthesize artificial nanosized latexes of poly(styrene-co-methyl methacrylate) or poly(styrene-co-ethyl methacrylate), which are in producing drug-releasing films.
- In the production of additive-manufactured methacrylate-based resins used in dentistry.
- In the synthesis of a star-shaped block copolymer electrolyte for all-solid-state lithium batteries.
- In the synthesis of a copolymer used as a matrix for semiconductor nanoparticles, which is crucial for the formation of a stable matrix for the quantum dots-copolymer composite material used in optoelectronic applications.
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
66.2 °F - closed cup
Punto de inflamabilidad (°C)
19 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico



![2-[3-(2H-Benzotriazol-2-il)-4-hidroxifenil]etilmetacrilato 99%](/deepweb/assets/sigmaaldrich/product/structures/208/967/cf29567e-c125-41dc-b80a-66889fa1a679/640/cf29567e-c125-41dc-b80a-66889fa1a679.png)