211672
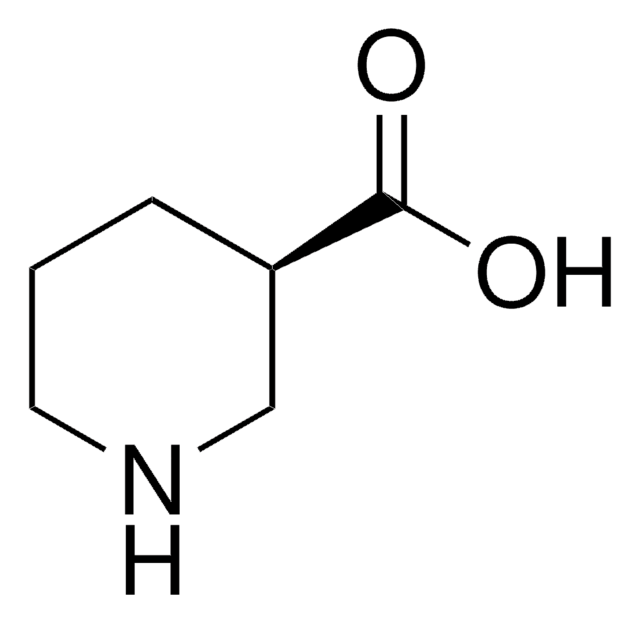
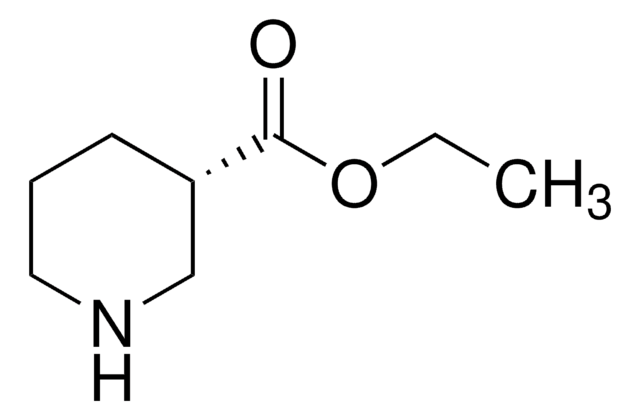
Nipecotic acid
98%
Sinónimos:
(±)-β-Homoproline, 3-Carboxypiperidine, 3-Piperidinecarboxylic acid, Hexahydronicotinic acid
About This Item
Productos recomendados
Análisis
98%
formulario
solid
mp
261 °C (dec.) (lit.)
solubilidad
water: soluble 50 mg/mL, clear, colorless to faintly yellow
cadena SMILES
OC(=O)C1CCCNC1
InChI
1S/C6H11NO2/c8-6(9)5-2-1-3-7-4-5/h5,7H,1-4H2,(H,8,9)
Clave InChI
XJLSEXAGTJCILF-UHFFFAOYSA-N
Información sobre el gen
human ... SLC23A2(9962)
Descripción general
Aplicación
Reactant for synthesis of:
Anticonvulsants
HCV NS5B polymerase inhibitors
Cathepsin S inhibitors
Orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation
Positive allosteric modulator of metabotropic glutamate receptor 4
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico