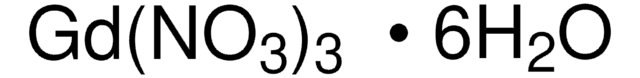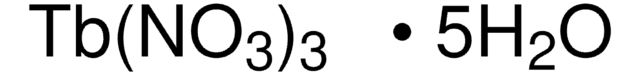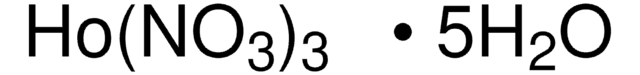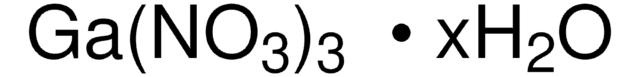211591
Gadolinium(III) nitrate hexahydrate
crystals and lumps, 99.9% trace metals basis
Sinónimos:
Gadolinium hexahydrate trinitrate
About This Item
Productos recomendados
Análisis
99.9% trace metals basis
formulario
crystals and lumps
idoneidad de la reacción
reagent type: catalyst
core: gadolinium
impurezas
≤1500.0 ppm Trace Rare Earth Analysis
mp
91 °C (lit.)
cadena SMILES
[Gd+3].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Gd.3NO3.6H2O/c;3*2-1(3)4;;;;;;/h;;;;6*1H2/q+3;3*-1;;;;;;
Clave InChI
XWFVFZQEDMDSET-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- As a precursor to synthesize Gadolinium oxide (Gd2O3) nanoparticles for various applications such as photocatalysis and biomedical imaging.
- As a dopant to prepare ZnO nanoparticles with enhanced room temperature ferromagnetism.
- To prepare Ceria (CeO2)-based ceramics doped electrolytes for intermediate temperature solid oxide fuel cells (IT-SOFCs) .
- As a raw material for the synthesis of Eu3+-Activated KGd2F7 red-emitting nanoparticles for white light-emitting diode.
- In addition,it is also reported to producegreen light emission of Er3+-activated single-phased GdAlO3 phosphors for lighting applications.
Otras notas
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
5.1B - Oxidizing hazardous materials
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico