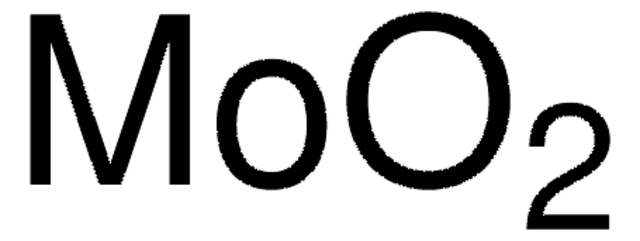203815
Molybdenum(VI) oxide
99.97% trace metals basis
Sinónimos:
Molybdenum trioxide
About This Item
Productos recomendados
Ensayo
99.97% trace metals basis
Formulario
powder
mp
795 °C (lit.)
aplicaciones
battery manufacturing
cadena SMILES
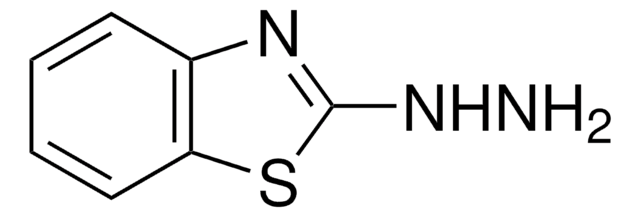
O=[Mo](=O)=O
InChI
1S/Mo.3O
Clave InChI
JKQOBWVOAYFWKG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Carc. 2 - Eye Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Nanostructured Materials Through Ultrasonic Spray Pyrolysis
The production of hydrogen by catalytic water splitting is important for a wide range of industries including renewable energy petroleum refining and for the production of methanol and ammonia in the chemical industry.
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico