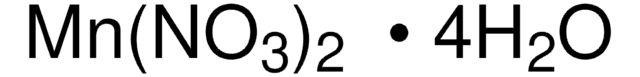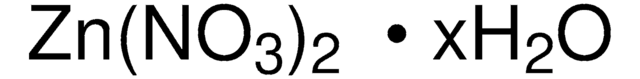203106
Cobalt(II) nitrate hexahydrate
99.999% trace metals basis
Sinónimos:
Cobaltous nitrate, Cobaltous nitrate hexahydrate, Nitric acid, cobalt(II) salt
About This Item
Productos recomendados
Análisis
99.999% trace metals basis
formulario
crystals and lumps
impurezas
≤15.0 ppm Trace Metal Analysis
mp
55 °C (lit.)
aplicaciones
battery manufacturing
cadena SMILES
O.O.O.O.O.O.[Co++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Co.2NO3.6H2O/c;2*2-1(3)4;;;;;;/h;;;6*1H2/q+2;2*-1;;;;;;
Clave InChI
QGUAJWGNOXCYJF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- Used as a precursor to synthesize nano-Co3O4 via direct thermal decomposition method for electrochemical water oxidation applications
- Used as a precursor to synthesize single-crystalline Co3O4 powders with controllable morphology for lithium-ion battery applications. cobalt-containing nanomaterials, such as nano-Co3O4, with controlled size, shape, and morphology via sol-gel method.
- As a vital precursor to generate nickel-rich cathode materials (NMC, NCA) for lithium-ion batteries using co-precipitation process because of its simplicity, ease of scale-up, and ability to produce a homogeneous structure at the particle scale.
Cobalt(II) nitrate hexahydrate can be used as a:
- A starting material in the preparation of Co-based nanomaterials and Co complexes.
- A catalyst to synthesize 5-carboxanilide-dihydropyrimidinone derivatives via a three-component condensation reaction.
- A dopant to prepare LaCr1−xCoxO3 solid-solution ceramics for high temperature applications.
Características y beneficios
- High purity with trace metal analysis (=< 15 ppm) for 32 elements, suitable for batteries.
- High water solubility ideal for synthesizing composites for various applications.
- Low ppm levels of metal ions including Al, K, Na, Mg, Cu, Co, etc.
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Ox. Sol. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1 - STOT RE 2 Inhalation
Órganos de actuación
Lungs
Código de clase de almacenamiento
5.1B - Oxidizing hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Nanostructured Materials Through Ultrasonic Spray Pyrolysis
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Thermoelectric Performance of Perovskite-type Oxide Materials
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico