196444
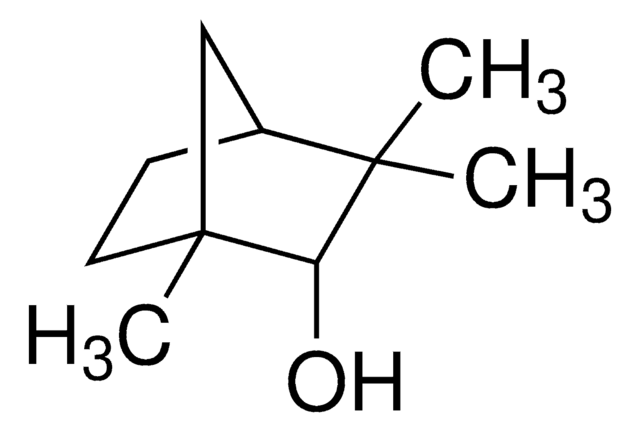
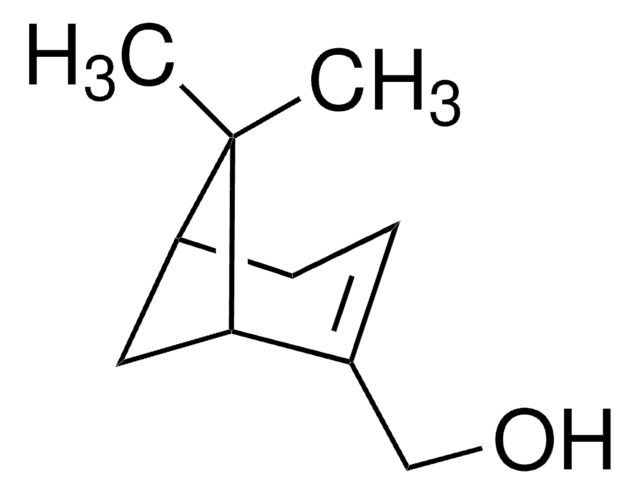
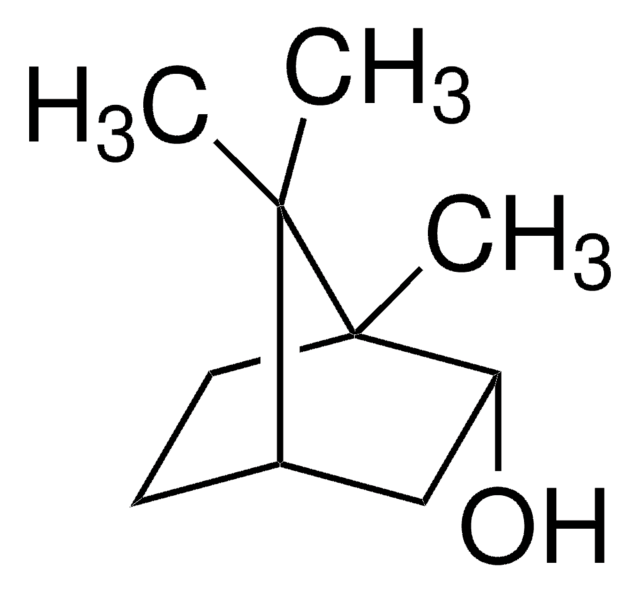
(1R)-endo-(+)-Fenchyl alcohol
96%
Sinónimos:
(+)-Fenchol, (1R)-1,3,3-Trimethylbicyclo[2.2.1]heptan-2-ol, 1,3,3-Trimethyl-2-norbornanol, Fenchyl alcohol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H18O
Número de CAS:
Peso molecular:
154.25
Beilstein:
2038082
Número MDL:
Código UNSPSC:
12352200
ID de la sustancia en PubChem:
Formulario:
solid
Ensayo:
96%
Productos recomendados
Nivel de calidad
Ensayo
96%
Formulario
solid
mp
39-45 °C (lit.)
grupo funcional
hydroxyl
cadena SMILES
CC1(C)[C@H]2CC[C@](C)(C2)[C@H]1O
InChI
1S/C10H18O/c1-9(2)7-4-5-10(3,6-7)8(9)11/h7-8,11H,4-6H2,1-3H3/t7-,8-,10+/m0/s1
Clave InChI
IAIHUHQCLTYTSF-OYNCUSHFSA-N
Descripción general
(1R)-endo-(+)-Fenchyl alcohol is a bicyclic monoterpenoid that contains a fenchane skeleton. It is a commonly used volatile compound in fragrances and flavoring agents.
Aplicación
(1R)-endo-(+)-Fenchyl alcohol can be used to prepare other terpenoids such as (+)-(1R,2S)-10-hydroxyfenchol, (+)-(1R,2R,3S)-8-hydroxyfenchol, (-)-(1S,2S,6S)-6-exo-hydroxyfenchol, and (-)-(1R,2R,3R)-9-hydroxyfenchol.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
165.2 °F - closed cup
Punto de inflamabilidad (°C)
74 °C - closed cup
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
M Miyazawa et al.
Xenobiotica; the fate of foreign compounds in biological systems, 37(9), 943-953 (2007-11-10)
The metabolism of (+)-fenchol was investigated in vitro using liver microsomes of rats and humans and recombinant cytochrome P450 (P450 or CYP) enzymes in insect cells in which human/rat P450 and NADPH-P450 reductase cDNAs had been introduced. The biotransformation of
Sergio Abbate et al.
Chirality, 21 Suppl 1, E242-E252 (2009-11-21)
The first well documented experiments of Near Infrared Vibrational Circular Dichroism (NIR-VCD) were performed around 1975. We review the thirty year history of NIR-VCD, encompassing both instrumental development and theoretical/computational methods that allow interpretation of experimental spectra, harvesting useful structural
D M Satterwhite et al.
The Journal of biological chemistry, 260(26), 13901-13908 (1985-11-15)
The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of this bound intermediate. To test this stereochemical scheme, phosphatase-free preparations of (-)-endo-fenchol cyclase from
D Yang et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 22(3), 128-131 (2003-02-11)
The essential oil isolated from the dried leaves of Lindera communis was analyzed by means of gas chromatography-mass(GC-MS) technique, the structures of 23 chemical components were identified from it in total, among these, (-)-spathulenol(relative content 22.50%), endo-1,3,3-trimethyl-2-norbornanol, acetate (10.06%), caryophyllene
R Croteau et al.
The Journal of biological chemistry, 263(30), 15449-15453 (1988-10-25)
The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of this bound intermediate. Incubation of (1R)-[2-14C,1-3H]- and (1S)-[2-14C,1-3H]geranyl pyrophosphate with a preparation of (-)-endo-fenchol
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico